Abstract
The purpose of this study was to examine whether the structural modification on the positively-charged Lys linker could reduce the kidney uptake of 99mTc-labeled Arg-Gly-Asp (RGD)-conjugated alpha-melanocyte stimulating hormone (α-MSH) hybrid peptides. The RGD motif {cyclic(Arg-Gly-Asp-DTyr-Asp)} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3–13 {(Arg11)CCMSH} through a neutral Glycine linker to eliminate the positively-charged amino side chain of Lys linker, or without linker to - delete the Lys linker. The receptor binding affinity of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH was determined in B16/F1 melanoma cells. The melanoma targeting and imaging properties of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 mice. The structural modification on the Lys linker retained low nanomolar receptor binding affinity of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH (1.5 and 1.0 nM, respectively). The structural modification on the Lys linker dramatically decreased the renal uptake of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH by 79% and 77% at 4 h post-injection compared to 99mTc-RGD-Lys-(Arg11)CCMSH. 99mTc-RGD-(Arg11)CCMSH displayed higher melanoma uptake (16.12 ± 3.09% ID/g) than 99mTc-RGD-Gly-(Arg11)CCMSH (11.50 ± 1.01% ID/g) at 2 post-injection. The tumor uptake of 99mTc-RGD-(Arg11)CCMSH was 1.4 times the tumor uptake of 99mTc-RGD-Gly-(Arg11)CCMSH at 2 post-injection. Dramatically enhanced tumor to kidney uptake ratio of 99mTc-RGD-(Arg11)CCMSH suggests that 188Re-RGD-(Arg11)CCMSH may behave in a similar fashion warranting future evaluation for melanoma treatment.
Keywords: RGD-conjugatedalpha-MSH hybrid peptide, Structural modification, Melanoma imaging
INTRODUCTION
Melanocortin-1 (MC1) receptor is a G protein-coupled receptor over-expressed on human and murine melanoma cells.1–6 Thus, melanoma cells can be differentiated from normal cells by targeting the higher levels of MC1 receptors on melanoma cells. Alpha-melanocyte stimulating hormone (α-MSH) peptides can bind to the MC1 receptors with low nanomolar binding affinities, highlighting the potential of using radiolabeled α-MSH peptides to bind the MC1 receptors for melanoma targeting. At present, radiolabeled linear, metal-cyclized and lactam bridge-cyclized α-MSH peptides have been utilized for melanoma imaging and therapy in melanoma-bearing mouse models.7–21 Recently, we have conjugated the RGD motif {cyclic(Arg-Gly-Asp-DTyr-Asp)} to the [Cys3,4,10, D-Phe7, Arg11]α-MSH3–13 {(Arg11)CCMSH} peptide through a Lys linker to yield RGD-Lys-(Arg11)CCMSH hybrid peptide for potential melanoma imaging and treatment.22 The (Arg11)CCMSH moiety was utilized for MC1 receptor binding and radiolabeling, whereas the RGD motif was used as an apoptosis inducer. RGD-Lys-(Arg11)CCMSH showed remarkable clonogenic cytotoxic effect in B16/F1 melanoma cells. Three-hour incubation with 0.1 μM of RGD-Lys-(Arg11)CCMSH decreased 65% of the clonogenic survival of B16/F1 cells compared to the untreated control cells six days post treatment. The biodistribution results of 99mTc-RGD-Lys-(Arg11)CCMSH showed high B16/F1 melanoma uptake of 14.83 ± 2.94% ID/g at 2 h post-injection, as well as extremely high non-specific renal uptake of 67.12 ± 8.79% ID/g at 2 h post-injection.22
From the therapeutic point of view, it is desirable to reduce the relatively high non-specific renal uptake ofα-MSH hybrid peptide. Thus, we managed to decrease the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH by substituting the Lys linker with the Arg linker.23 Interestingly, we found that the replacement of the Lys linker with the Arg linker dramatically improved the melanoma uptake by 44% (from 14.83 ± 2.94 to 21.41 ± 3.74% ID/g) and reduced the renal uptake by 36% (from 67.12 ± 8.79 to 43.01 ± 8.14% ID/g) at 2 h post-injection.23 Furthermore, co-injection of 15 mg of L-lysine substantially decreased the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH by 52% (from 67.12 ± 8.79 to 32.20 ± 5.98% ID/g) and the renal uptake of 99mTc-RGD-Arg-(Arg11)CCMSH by 28% (from 43.01 ± 8.14 to 31.10 ± 6.42% ID/g) at 2 h post-injection, indicating that the overall positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH substantially contributed to their non-specific renal uptake.23 Therefore, we hypothesized that the elimination of the positive charge associated with the side chain of Lys or Arg linker would decrease the non-specific renal uptake of 99mTc-labeled RGD-conjugated α-MSH hybrid peptide.
To examine our hypothesis, we synthesized two novel RGD-conjugated α-MSH hybrid peptides named RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH in this study. The RGD motif was coupled to the (Arg11)CCMSH peptide through the neutral Gly linker or without any linker. The substitution of the Lys linker with Gly linker eliminated the positively-charged amino side chain of Lys, whereas the direct coupling of the RGD motif to the (Arg11)CCMSH peptide deleted the Lys linker. We determined the receptor binding affinities of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH in B16/F1 melanoma cells. Meanwhile, we examined the melanoma targeting and imaging properties of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice.
EXPERIMENTAL SECTION
Chemicals and Reagents
Amino acids and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). 125I-Tyr2-[Nle4, D-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Shelton, CT) for in vitro receptor binding assay. 99mTcO4− was purchased from Cardinal Health (Albuquerque, NM) for peptide radiolabeling. All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Peptide Synthesis
New RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH hybrid peptides were synthesized on Sieber amide resin using 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY) according to our published procedure22 with modifications. Briefly, 70 μmol of Sieber amide resin and 210 μmol of Fmoc-protected amino acids were used for the synthesis. Fmoc-Gly was used to generate the Gly linker in the hybrid peptide. RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by liquid chromatography-mass spectroscopy (LC-MS).
In vitro Receptor Binding Assay
The IC50 values of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were determined in B16/F1 melanoma cells. The receptor binding assay was replicated in triplicate for each peptide. Briefly, the B16/F1 cells in 24-well cell culture plates (5×105/well) were incubated at room temperature (25°C) for 2 h with approximately 40,000 counts per minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of increasing concentrations (10−12 to 10−5 M) of either RGD-Gly-(Arg11)CCMSH or RGD-(Arg11)CCMSH in 0.3 mL of binding medium {Modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}. The medium was aspirated after the incubation. The cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA/0.01 M phosphate buffered saline (PBS) and lysed in 0.5 mL of 1 N NaOH for 5 minutes. The activities associated with cells were measured in a Wallac 1480 automated gamma counter (PerkinElmer, Waltham, MA). The IC50 values were calculated using Prism software (GraphPad Software, La Jolla, CA).
Peptide Radiolabelling
The RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were radiolabeled with 99mTc via a glucoheptonate transchelation reaction according to our published procedure.22 Briefly, 99mTcO4− (~74 MBq) was reduced by SnCl2 to form 99mTc-glucoheptonate at 25 °C in the first step. Then 10 μg of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH was added into the reaction vial to compete off the glucoheptonate at 75 °C to yield 99mTc-RGD-Gly-(Arg11)CCMSH or 99mTc-RGD-(Arg11)CCMSH. For stability, biodistribution and imaging studies, each radiolabeled peptide was purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytical column (Deerfield, IL) using a 20-min gradient of 16–26% acetonitrile in 20 mM HCl aqueous solution at an 1 mL/min flow rate. Each purified peptide was purged with N2 gas for 20 mins to remove the acetonitrile. The pH of final peptide solution was adjusted to 7.4 with 0.1 N NaOH and sterile normal saline for stability, biodistribution and imaging studies. The serum stability of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH was determined by incubation in mouse serum at 37 °C for 24 h and monitored for degradation by RP-HPLC. Briefly, 100 μL of HPLC-purified peptide solution (~7.4 MBq) was added into 100 μL of mouse serum (Sigma-Aldrich Corp, St. Louis, MO) and incubated at 37°C for 24 h. After the incubation, 200 μL of a mixture of ethanol and acetonitrile (V:V = 1:1) was added to precipitate the serum. The resulting mixture was centrifuged at 10,000 g for 5 min to collect the supernatant. The supernatant was purged with N2 gas for 30 min to remove the ethanol and acetonitrile. The resulting sample was mixed with 500 μL of water and injected into RP-HPLC for analysis using the gradient described above.
Biodistribution and Melanoma Imaging Studies
All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The biodistribution properties of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). The C57 mice were subcutaneously inoculated on the right flank with 1×106 B16/F1 cells. The weight of tumors reached approximately 0.2 g 10 days post cell inoculation. Each melanoma-bearing mouse was injected with 0.037 MBq of 99mTc-RGD-Gly-(Arg11)CCMSH or 99mTc-RGD-(Arg11)CCMSH via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the body weight. The specificity of tumor uptake was determined by co-injecting 99mTc-RGD-Gly-(Arg11)CCMSH or 99mTc-RGD-(Arg11)CCMSH with 10 μg (6.1 nmol) of unlabeled NDP-MSH at 2 h post-injection.
To determine the melanoma imaging properties, approximately 4.4 MBq of 99mTc-RGD-Gly-(Arg11)CCMSH or 4.1 MBq of 99mTc-RGD-(Arg11)CCMSH was injected into two B16/F1 melanoma-bearing C57 mice via the tail vein, respectively. The mice were euthanized for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan, Washington DC) imaging 2 h post-injection. The 9-min CT imaging was immediately followed by the SPECT imaging of whole-body. The SPECT scans of 24 projections were acquired. Reconstructed data from SPECT and CT were visualized and co-registered using InVivoScope (Bioscan, Washington DC).
Statistical Analysis
Statistical analysis was performed using the Student’s t-test for unpaired data to determine the significance of differences in tumor uptake with or without peptide blockade in biodistribution studies described above. Differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
New RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were synthesized and purified by RP-HPLC. The schematic structures of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH are presented in Figure 1. The structures of RGD-Lys-(Arg11)CCMSH and RGD-Arg-(Arg11)CCMSH were cited from our previous publications22,23 for comparison. The peptide identities were confirmed by electrospray ionization mass spectrometry. The chemical purities of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were greater than 95%. The molecular weights of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH are shown in Table 1. The measured molecular weights of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were 2080 and 2022, respectively. The IC50 values of RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH were 1.5 and 1.0 nM in B16/F1 melanoma cells (Table 1).
Figure 1.
Schematic structures of RGD-Gly-(Arg11)CCMSH, RGD-(Arg11)CCMSH, RGD-Lys-(Arg11)CCMSH and RGD-Arg-(Arg11)CCMSH. The structures of RGD-Lys-(Arg11)CCMSH and RGD-Arg-(Arg11)CCMSH were cited from our previous reports 22,23 for comparison.
Table 1.
IC50 values and molecular weights (MW) of RGD-Gly-(Arg11)CCMSH, RGD-(Arg11)CCMSH, RGD-Lys-(Arg11)CCMSH and RGD-Arg-(Arg11)CCMSH.
The peptides were readily labeled with 99mTc with greater than 95% radiolabeling yield. 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were completely separated from their excess non-labeled peptides by RP-HPLC. The retention times of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were 17.5 and 19.9 min, respectively. Both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were stable in mouse serum at 37 °C for 24 h. Only 99mTc-RGD-Gly-(Arg11)CCMSH or 99mTc-RGD-(Arg11)CCMSH was detected by RP-HPLC. The melanoma targeting and pharmacokinetic properties of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 mice. The biodistribution results of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH are presented in Tables 2 and 3. 99mTc-RGD-Gly-(Arg11)CCMSH exhibited rapid and high tumor uptake in melanoma-bearing mice. The tumor uptake was 9.44± 1.22% ID/g at 0.5 h post-injection. 99mTc-RGD-Gly-(Arg11)CCMSH reached its peak tumor uptake of 11.50 ± 1.01% ID/g at 2 h post-injection. The tumor uptake of 99mTc-RGD-Gly-(Arg11)CCMSH decreased to 7.99± 1.05% ID/g at 4 h post-injection, and gradually decreased to 5.55± 0.89% ID/g at 24 h post-injection. Seventy eight percent of the tumor uptake of 99mTc-RGD-Gly-(Arg11)CCMSH was blocked with 10 μg (6.1 nmol) of non-radiolabeled NDP-MSH at 2 h post-injection (p<0.05), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Whole-body clearance of 99mTc-RGD-Gly-(Arg11)CCMSH was rapid, with approximately 73% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 2). Normal organ uptake of 99mTc-RGD-Gly-(Arg11)CCMSH was lower than 3.5% ID/g except for the kidneys after 2 h post-injection. High tumor/blood and tumor/muscle uptake ratios were demonstrated as early as 2 h post-injection (Table 2). The renal uptake of 99mTc-RGD-Gly-(Arg11)CCMSH reached its peak value of 33.69 ± 12.04% ID/g at 0.5 h post-injection, and gradually decreased to 3.23 ± 1.01% ID/g at 24 h post-injection.
Table 2.
Biodistribution of 99mTc-RGD-Gly-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice. The data was presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5).
| Tissue | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP Blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 9.44 ± 1.22 | 11.50 ± 1.01 | 7.99 ± 1.05 | 5.55 ± 0.89 | 2.57 ± 1.39* |
| Brain | 0.16 ± 0.01 | 0.07 ± 0.02 | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.09 ± 0.08 |
| Blood | 5.07 ± 3.40 | 0.70 ± 0.47 | 0.71 ± 0.72 | 0.32 ± 0.44 | 0.44 ± 0.27 |
| Heart | 2.56 ± 0.50 | 0.71 ± 0.10 | 0.51 ± 0.20 | 0.20 ± 0.05 | 1.36 ± 1.41 |
| Lung | 7.49 ± 2.03 | 1.67 ± 0.71 | 1.13 ± 0.44 | 0.34 ± 0.11 | 2.84 ± 2.62 |
| Liver | 3.44 ± 0.73 | 2.52 ± 0.41 | 2.75 ± 0.51 | 0.72 ± 0.14 | 2.55 ± 1.38 |
| Skin | 6.39 ± 0.84 | 1.56 ± 0.43 | 1.00 ± 0.39 | 0.34 ± 0.16 | 1.11 ± 0.54 |
| Spleen | 2.32 ± 1.10 | 0.94 ± 0.31 | 1.18 ± 0.43 | 0.89 ± 0.04 | 1.28 ± 0.68 |
| Stomach | 3.87 ± 1.00 | 3.43 ± 0.69 | 2.94 ± 0.88 | 0.67 ± 0.11 | 2.88 ± 0.66 |
| Kidneys | 33.69 ± 12.04 | 20.85 ± 4.51 | 14.81 ± 5.41 | 3.23 ± 1.01 | 15.47 ± 3.39* |
| Muscle | 1.02 ± 0.70 | 0.57 ± 0.29 | 0.11 ± 0.09 | 0.30 ± 0.08 | 0.43 ± 0.29 |
| Pancreas | 1.74 ± 0.29 | 0.33 ± 0.11 | 0.24 ± 0.13 | 0.31 ± 0.15 | 0.42 ± 0.21 |
| Bone | 2.02 ± 0.51 | 0.87 ± 0.15 | 0.50 ± 0.30 | 0.47 ± 0.12 | 1.18 ± 1.07 |
| Percent injected dose (%ID) | |||||
| Intestines | 3.41 ± 0.23 | 2.41 ± 0.42 | 4.71 ± 2.80 | 1.44 ± 0.78 | 2.49 ± 1.10 |
| Urine | 38.34 ± 8.90 | 72.97 ± 9.29 | 78.79 ± 3.38 | 92.74 ± 1.99 | 69.61 ± 23.76 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/Blood | 1.86 | 16.43 | 11.25 | 17.34 | 5.84 |
| Tumor/Kidneys | 0.28 | 0.55 | 0.54 | 1.72 | 0.17 |
| Tumor/Lung | 1.26 | 6.89 | 7.07 | 16.32 | 0.90 |
| Tumor/Liver | 2.74 | 4.56 | 2.91 | 7.71 | 1.01 |
| Tumor/Muscle | 9.25 | 20.18 | 72.64 | 18.50 | 5.98 |
p<0.05, significance of difference in tumor and renal uptake between 99mTc-RGD-Gly-(Arg11)CCMSH with or without peptide blockade at 2 h post-injection.
Table 3.
Biodistribution of 99mTc-RGD-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice. The data was presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5).
| Tissue | 0.5 h | 2 h | 4 h | 24 h | 2 h NDP Blockade |
|---|---|---|---|---|---|
| Percent injected dose/gram (%ID/g) | |||||
| Tumor | 9.46 ± 1.07 | 16.12 ± 3.09 | 12.10 ± 1.45 | 5.74 ± 1.22 | 2.55 ± 0.07* |
| Brain | 0.21 ± 0.10 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.02 | 0.05 ± 0.02 |
| Blood | 6.10 ± 5.05 | 0.74 ± 0.34 | 0.48 ± 0.32 | 0.08 ± 0.07 | 0.44 ± 0.37 |
| Heart | 3.09 ± 1.13 | 0.70 ± 0.15 | 0.31 ± 0.08 | 0.08 ± 0.03 | 0.41 ± 0.16 |
| Lung | 8.24 ± 2.27 | 1.54 ± 0.20 | 0.63 ± 0.29 | 0.24 ± 0.08 | 1.13 ± 0.22 |
| Liver | 2.73 ± 0.75 | 2.47 ± 0.20 | 1.55 ± 0.25 | 0.65 ± 0.10 | 1.21 ± 0.24 |
| Skin | 7.49 ± 1.75 | 1.25 ± 0.31 | 0.53 ± 0.07 | 0.32 ± 0.13 | 0.99 ± 0.35 |
| Spleen | 1.66 ± 0.25 | 1.06 ± 0.39 | 0.66 ± 0.31 | 0.57 ± 0.25 | 0.75 ± 0.21 |
| Stomach | 4.49 ± 1.59 | 1.52 ± 0.24 | 0.85 ± 0.19 | 0.69 ± 0.25 | 2.24 ± 0.55 |
| Kidneys | 29.34 ± 2.98 | 23.88 ± 1.45 | 16.01 ± 2.47 | 2.80 ± 0.84 | 13.32 ± 1.68* |
| Muscle | 0.98 ± 0.21 | 0.20 ± 0.11 | 0.20 ± 0.02 | 0.22 ± 0.15 | 0.24 ± 0.03 |
| Pancreas | 1.02 ± 0.52 | 0.29 ± 0.10 | 0.20 ± 0.07 | 0.12 ± 0.07 | 0.19 ± 0.11 |
| Bone | 3.03 ± 0.97 | 0.92 ± 0.35 | 0.59 ± 0.18 | 0.36 ± 0.27 | 0.59 ± 0.20 |
| Percent injected dose (%ID) | |||||
| Intestines | 3.80 ± 0.32 | 1.76 ± 0.37 | 1.35 ± 0.24 | 1.22 ± 0.21 | 3.86 ± 0.84 |
| Urine | 29.29 ± 12.36 | 74.6 ± 4.55 | 85.92 ± 2.09 | 90.19 ± 3.25 | 84.07 ± 1.64 |
| Uptake ratio of tumor/normal tissue | |||||
| Tumor/Blood | 1.55 | 21.78 | 25.21 | 71.75 | 5.80 |
| Tumor/Kidneys | 0.32 | 0.68 | 0.76 | 2.05 | 0.19 |
| Tumor/Lung | 1.15 | 10.47 | 19.21 | 23.92 | 2.26 |
| Tumor/Liver | 3.47 | 6.53 | 7.81 | 8.83 | 2.11 |
| Tumor/Muscle | 9.65 | 80.60 | 60.50 | 26.09 | 10.63 |
p<0.05, significance of difference in tumor and renal uptake between 99mTc-RGD-(Arg11)CCMSH with or without peptide blockade at 2 h post-injection.
99mTc-RGD-(Arg11)CCMSH exhibited similar biodistribution pattern as 99mTc-RGD-Gly-(Arg11)CCMSH. Specifically, 99mTc-RGD-(Arg11)CCMSH displayed similar renal uptake at all time points investigated, and slightly higher tumor uptake at 2 and 4 h post-injection (Tables 2 and 3). The tumor uptake of 99mTc-RGD-(Arg11)CCMSH was 9.46± 1.07% ID/g at 0.5 h post-injection. 99mTc-RGD-(Arg11)CCMSH reached its peak tumor uptake value of 16.12± 3.09% ID/g at 2 h post-injection. The tumor uptake of 99mTc-RGD-(Arg11)CCMSH was 12.10± 1.45% ID/g at 4 h post-injection, and gradually decreased to 5.74± 1.22% ID/g at 24 h post-injection. Eighty four percent of the tumor uptake of 99mTc-RGD-(Arg11)CCMSH was blocked with 10 μg (6.1 nmol) of non-radiolabeled NDP-MSH at 2 h post-injection (p<0.05), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Whole-body clearance of 99mTc-RGD-(Arg11)CCMSH was fast, with approximately 75% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 3). Normal organ uptake of 99mTc-RGD-(Arg11)CCMSH was lower than 2.5% ID/g except for the kidneys after 2 h post-injection. High tumor/blood and tumor/muscle uptake ratios were demonstrated as early as 2 h post-injection (Table 3). The renal uptake of 99mTc-RGD-(Arg11)CCMSH reached its peak value of 29.34 ± 2.98% ID/g at 0.5 h post-injection, and gradually decreased to 2.80 ± 0.84% ID/g at 24 h post-injection.
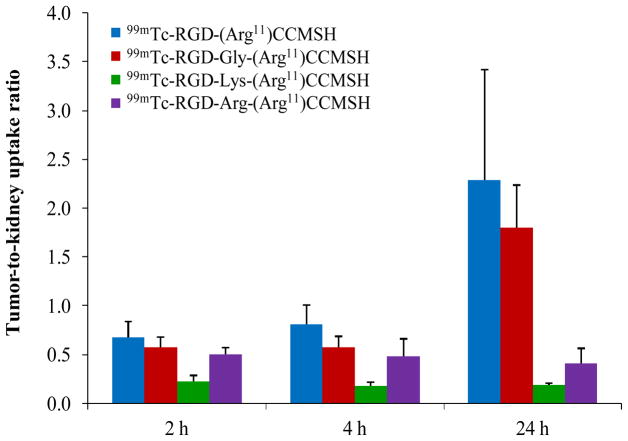
The tumor to kidney uptake ratios of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH are summarized and compared with 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH in Figure 2. 99mTc-RGD-Gly-(Arg11)CCMSH showed higher tumor to kidney uptake ratios than 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH at 2, 4 and 24 h post-injection. 99mTc-RGD-(Arg11)CCMSH exhibited the highest tumor to kidney uptake ratios among the 99mTc-labeled hybrid peptides at 2, 4 and 24 h post-injection. Two B16/F1 melanoma-bearing C57 mice were injected with 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH through the tail vein to visualize the tumors 2 h after dose administration. The whole-body SPECT/CT images are presented in Figure 3. Flank melanoma tumors were visualized clearly by both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH at 2 h post-injection. Both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH exhibited high tumor to normal organ uptake ratios except for the kidney.
Figure 2.
Tumor to kidney uptake ratios of 99mTc-RGD-(Arg11)CCMSH, 99mTc-RGD-Gly-(Arg11)CCMSH, 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH at 2, 4 and 24 h post-injection. The tumor to kidney uptake ratios of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH were calculated based on our previous reports 22,23 for comparison.
Figure 3.

Whole-body SPECT/CT images of 99mTc-RGD-(Arg11)CCMSH (A) and 99mTc-RGD-Gly-(Arg11)CCMSH (B) in B16/F1 melanoma-bearing C57 mice at 2 h post-injection. Tumor (T) lesions were highlighted with arrows on the images.
DISCUSSION
Melanoma metastases are very aggressive, leading to high mortality of malignant melanoma. Unfortunately, no curative treatment is available for metastatic melanoma. Thus, novel and effective therapeutic approaches are urgently needed to fulfill the desperate need for melanoma treatment. Recently, we have successfully utilized the MC1 receptor-targeting (Arg11)CCMSH peptide to target the RGD motif to melanoma cells to induce apoptosis.22,23 Both 100 nM of RGD-Lys-(Arg11)CCMSH or RGD-Arg-(Arg11)CCMSH exhibited remarkable growth inhibition in B16/F1 melanoma cells. However, high renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH needed to be reduced to facilitate their therapeutic applications. As we previously reported, the switch from the Lys linker to the Arg linker dramatically decreased the renal uptake by 36% at 2 h post-injection.23 Meanwhile, we found that the overall positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH substantially contributed to their non-specific renal uptake.23 Thus, we were interested in examining whether the reduction of the overall positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH could further reduce their renal uptake in this study.
It is worthwhile to note that there are three positively-charged arginines in RGD-Lys-(Arg11)CCMSH or RGD-Arg-(Arg11)CCMSH peptides besides the positively-charged Lys or Arg linker. Three arginines together with Lys/Arg linker contributed to the overall positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH. Hence, it was possible to reduce the overall positive charges of 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-RGD-Arg-(Arg11)CCMSH by altering either positively-charged arginines or Lys/Arg linker. However, we have demonstrated that the three arginines were critical for MC1 or αvβ3 receptor binding of the hybrid peptide.6 Therefore, we modified the amino acid linker (rather than three arginines) of the hybrid peptide by two means in this study. Firstly, we replaced the Lys linker with Gly linker to eliminate the positively-charged amino side chain of Lys to generate RGD-Gly-(Arg11)CCMSH peptide. Secondly, we directly conjugated the RGD motif to (Arg11)CCMSH to completely delete the Lys linker to yield RGD-(Arg11)CCMSH peptide. The structural modifications on the Lys linker retained low nanomolar MC1 receptor binding affinities of the hybrid peptides. RGD-Gly-(Arg11)CCMSH displayed comparable IC50 value as RGD-Lys-(Arg11)CCMSH (1.5 vs. 2.1 nM22), whereas RGD- (Arg11)CCMSH showed similar IC50 value as RGD-Arg-(Arg11)CCMSH (1.0 vs. 0.7 nM23).
In our previous report,23 the substitution of Lys linker with Arg linker decreased the renal uptake. 99mTc-RGD-Arg-(Arg11)CCMSH exhibited 36% less renal uptake than 99mTc-RGD-Lys-(Arg11)CCMSH at 2 h post-injection.23 In this study, the elimination of the positively-charged amino side chain of Lys linker or completely deletion of Lys linker dramatically decreased the renal uptake of 99mTc-labeled RGD-Gly-(Arg11)CCMSH and RGD-(Arg11)CCMSH in B16/F1 melanoma-bearing C57 mice. Compared to 99mTc-RGD-Lys-(Arg11)CCMSH, both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH exhibited similar lower renal uptake at all time points investigated. The renal uptake of 99mTc-RGD-(Arg11)CCMSH was 42, 35, 23 and 7% of the renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH at 0.5, 2, 4 and 24 h post-injection, respectively. As we anticipated, 99mTc-RGD-(Arg11)CCMSH displayed higher tumor uptake than 99mTc-RGD-Gly-(Arg11)CCMSH at 2 and 4 h post-injection. The tumor uptake of 99mTc-RGD-(Arg11)CCMSH was 1.4 and 1.5 times the tumor uptake of 99mTc-RGD-Gly-(Arg11)CCMSH at 2 and 4 h post-injection. A dramatic decrease in the renal uptake of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH resulted in enhanced tumor to kidney uptake ratios. Both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH exhibited higher tumor to kidney uptake ratios than 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-Arg-(Arg11)CCMSH at 2, 4 and 24 h post-injection (Fig. 2). The tumor to kidney uptake ratio of 99mTc-RGD-Gly-(Arg11)CCMSH was 2.5, 3.2 and 9.5 times the tumor to kidney uptake ratio of 99mTc-RGD-Lys-(Arg11)CCMSH at 2, 4 and 24 h post-injection, respectively. Among these four 99mTc-labeled RGD-conjugated α-MSH hybrid peptides, 99mTc-RGD-(Arg11)CCMSH exhibited the highest tumor to kidney uptake ratios (Fig. 2). The tumor to kidney uptake ratio of 99mTc-RGD-(Arg11)CCMSH was 3.8, 4.5 and 12.1 times the tumor to kidney uptake ratio of 99mTc-RGD-Lys-(Arg11)CCMSH at 2, 4 and 24 h post-injection, respectively. Both 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH (Fig. 3) displayed substantial tumor to kidney imaging contrast in this study.
From therapeutic perspective, the improved tumor to kidney uptake ratio of 99mTc-RGD-(Arg11)CCMSH will facilitate further therapeutic evaluation of RGD-(Arg11)CCMSH peptide when labeled with therapeutic 188Re. One advantage associated with RGD-(Arg11)CCMSH hybrid peptide is that the peptide can be readily labeled with 188Re without any structural modification because 188Re shares similar coordination chemistry with 99mTc. Beta-particle-emitting 188Re (T1/2=16.95 h, Eβmax=2.11 MeV) can be easily obtained from a 188W/188Re generator, which can be shipped to research laboratories for routine practice. The high energies and long path-lengths of beta-particles make 188Re be suited for large tumors. The cross-fire effects of 188Re can establish a homogeneous radiation field in the tumor that can overcome receptor expression heterogeneity. Hence, it will be attractive to determine the therapeutic efficacy of 188Re-RGD-(Arg11)CCMSH for melanoma in the future. Meanwhile, despite the significant reduction of renal uptake reported in this study, further work is clearly needed to reduced potential renal toxicity resulting from the therapeutic use of this class of peptides.
In conclusion, the structural modification on the Lys linker exhibited a profound effect on reducing the renal uptake of 99mTc-labeled RGD-conjugated α-MSH hybrid peptides. Either elimination of the positively-charged amino side chain of Lys linker or deletion of Lys linker dramatically decreased the renal uptake of 99mTc-RGD-Gly-(Arg11)CCMSH and 99mTc-RGD-(Arg11)CCMSH by 79% and 77% at 4 h post-injection compared to 99mTc-RGD-Lys-(Arg11)CCMSH. 99mTc-RGD-(Arg11)CCMSH displayed higher melanoma uptake than 99mTc-RGD-Gly-(Arg11)CCMSH at 2 and 4 h post-injection. The tumor uptake of 99mTc-RGD-(Arg11)CCMSH was 1.4 and 1.5 times the tumor uptake of 99mTc-RGD-Gly-(Arg11)CCMSH at 2 and 4 h post-injection. Dramatically enhanced tumor to kidney ratio of 99mTc-RGD-(Arg11)CCMSH suggests that 188Re-RGD-(Arg11)CCMSH may behave in a similar fashion warranting future evaluation for melanoma treatment..
Acknowledgments
We appreciate Dr. Fabio Gallazzi for technical assistance. This work was supported in part by the NIH grant NM-INBRE P20RR016480/P20GM103451, University of New Mexico STC Gap Fund and University of New Mexico HSC RAC Award. The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
References
- 1.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 3.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mtechnetium-labeled cyclic alpha-melanocyte-stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- 4.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Bioconjug Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 5.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Metastatic melanoma imaging with an 111In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide. Nucl Med Biol. 2009;36:267–276. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Guo H, Miao Y. Technetium-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptides for human melanoma imaging. Nucl Med Biol. 2010;37:873–883. doi: 10.1016/j.nucmedbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, Quinn TP. Evaluation of an 111In-DOTA-rhenium cyclized α-MSH analog: a novel cyclic-peptide analog with improved tumor-targeting properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- 8.Chen J, Cheng Z, Miao Y, Owen NK, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: synthesis and biological evaluation of 111In-labeled DOTA-conjugated rhenium-cyclized alpha-MSH analogues. J Med Chem. 2002;45:3048–3056. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 9.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 10.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 11.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A Gallium-labeled DOTA-α-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 12.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. J Nucl Med. 2005;46:887–895. [PubMed] [Google Scholar]
- 13.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–129. [PubMed] [Google Scholar]
- 14.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore HA, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman TJ, Quinn TP. Melanoma therapy via peptide-targeted alpha-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 15.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Lewis JS. Synthesis and biologic evaluation of 64Cu-labeled rhenium-cyclized alpha-MSH peptide analog using a cross-bridged cyclam chelator. J Nucl Med. 2007;48:64–72. [PubMed] [Google Scholar]
- 16.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 17.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for MicroPET imaging of melanocortin 1 receptor expression. Bioconjug Chem. 2007;18:765–772. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjug Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H, Yang J, Gallazzi F, Prossnitz ER, Sklar LA, Miao Y. Effect of DOTA position on melanoma targeting and pharmacokinetic properties of 111In-labeled lactam bridge-cyclized α-melanocyte stimulating hormone peptide. Bioconjug Chem. 2009;20:2162–2168. doi: 10.1021/bc9003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo H, Yang J, Shenoy N, Miao Y. Gallium-67-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide for primary and metastatic melanoma imaging. Bioconjug Chem. 2009;20:2356–2363. doi: 10.1021/bc900428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide resulting in enhanced melanoma uptake. J Nucl Med. 2010;51:418–426. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Guo H, Gallazzi F, Berwick M, Padilla RS, Miao Y. Evaluation of a novel RGD-conjugated alpha-melanocyte stimulating hormone hybrid peptide for potential melanoma therapy. Bioconjug Chem. 2009;20:1634–1642. doi: 10.1021/bc9001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Guo H, Padilla RS, Berwick M, Miao Y. Replacement of the Lys linker with an Arg linker resulting in improved melanoma uptake and reduced renal uptake of Tc-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptide. Bioorg Med Chem. 2010;18:6695–6700. doi: 10.1016/j.bmc.2010.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]