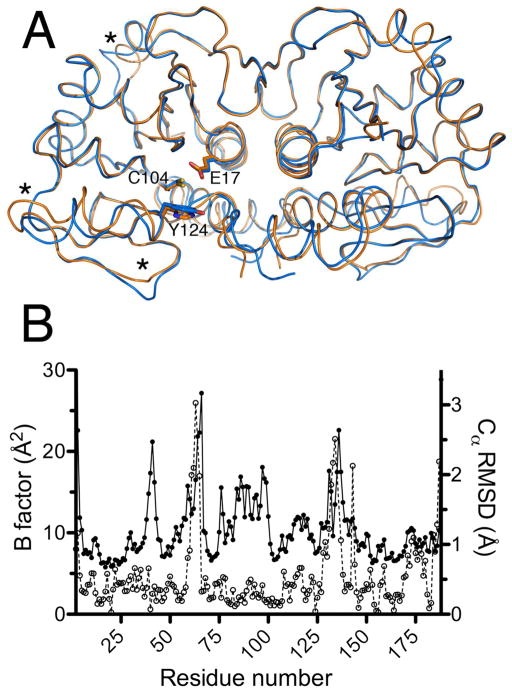
Figure 1.
DJ-1β and human DJ-1 are structurally similar. Panel A: The physiologically relevant dimeric forms of human DJ-1 (orange) and Drosophila DJ-1β (blue) are superimposed, showing nearly identical backbone structures. The functionally significant residues Cys104 (Cys106), Glu17 (Glu18) and Tyr124 (His126) are shown (human DJ-1 residue numbering in parenthesis). Regions with the largest variation between the human and fly proteins are marked with asterisks. Panel B: The areas of greatest structural variation between the human and Drosophila proteins correlate with elevated atomic displacement parameter values in the human protein. Filled circles represent the average equivalent isotropic B factors for the Cα atoms of human DJ-1 (PDB 2OR3), while open circles represent the r.m.s.d. of the Cα atoms of the Drosophila and human DJ-1 crystal structures. The areas marked with asterisks in panel A correspond to the r.m.s.d. peaks from residues 60-70 and 125-150. In both plots, only data for chain A of the dimeric proteins are shown.