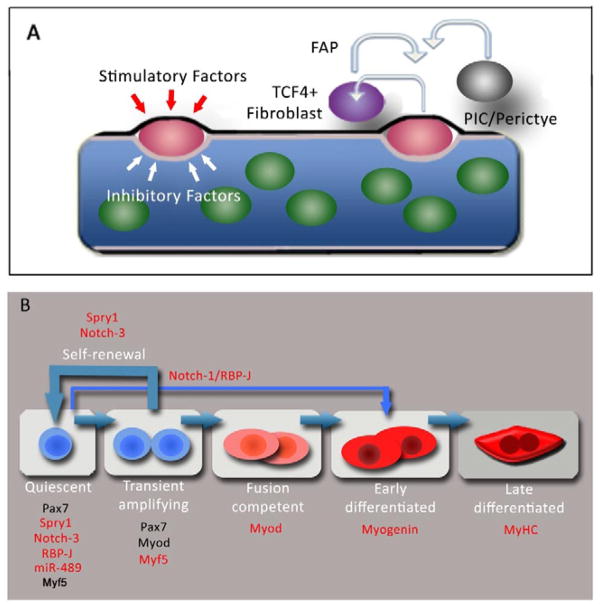
Figure 4. Regulation of MuSC functions in satellite cells.
(A) The MuSC niche. The satellite cell (red) resides in a quiescent state in contact with the plasmalemma (brown) of the myofiber and basal lamina (black) in a milieu of inhibitory (white arrows) and stimulatory factors (red arrows) that retain satellite cells in a stem cell state and inhibit their differentiation. Other cells in close proximity to satellite cells in vivo such as TCF4+ fibroblasts, FAPs, PICs and pericytes may constitute components of the MuSC niche and signal to the satellite cell and vice versa. (B) Schematic of MuSC regulation. Satellite cells transition from quiescence into a transient amplification stage most progress along a myogenic lineage to differentiate while a subset self renew and return back to quiescence (thick blue arrows). Markers denoting each stage of lineage progression are shown in black, molecules required for distinct satellite cell functions are shown in red. Quiescent satellite cells express Spry1, Notch-3 and miR-489. RBP-J and Myf5 is expressed in quiescent and cycling satellite cells. RBP-J and miR-489 (red) are required to retain satellite cells in quiescence. Spry1 and Notch-3 are required in cycling satellite cells for their return to quiescence and homeostasis of satellite cell pool after injury. Myf5 is required for normal transient amplification of progenitors. Myod, Myogenin and Myosin Heavy Chain (MyHC) are required for differentiation. Normal step-wise lineage progression of satellite cells depends on RBP-J (thin blue arrow), possibly through modulation of Notch-1 signaling.