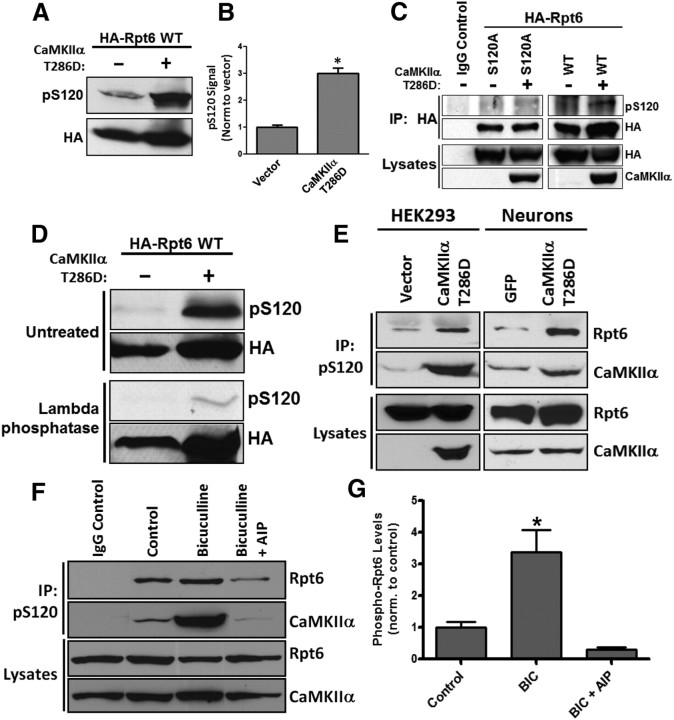
Figure 1.
Rpt6 is phosphorylated at S120 by CaMKIIα. A, B, Lysates from HEK293 cells expressing either HA-Rpt6 WT alone or HA-Rpt6 WT plus CaMKIIα T286D showed an approximately threefold increase in Rpt6 pS120 immunoreactive signal in coexpressing cells (n = 4; p < 0.001, unpaired Student's t test). C, When expressed in HEK293 cells alone or together with CaMKIIα, Rpt6 S120A has no pS120 immunoreactivity. D, λ-Phosphatase treatment of nitrocellulose membranes efficiently removed pS120 immunoreactivity. E, Expression of CaMKIIα T286D in HEK293 cells (transfection) and neurons (viral transduction) increased phosphorylation of endogenous Rpt6. Representative blot from three experiments. F, G, Increased phosphorylation of Rpt6 at S120 and enhanced association of Rpt6 and CaMKIIα in neurons treated with BIC (20 μm, 24 h), but not in cells treated with BIC and the CaMKII inhibitor AIP (2 μm). Immunoprecipitates (using Rpt6 pS120 antibody) from lysates of treated neuronal cultures were analyzed by SDS-PAGE. The blots were then probed with Rpt6 (mAb) and CaMKIIa (mAb) antibodies. n = 3–6 individual experiments; one-way ANOVA with post hoc Bonferroni's multiple-comparison test, *p < 0.05).