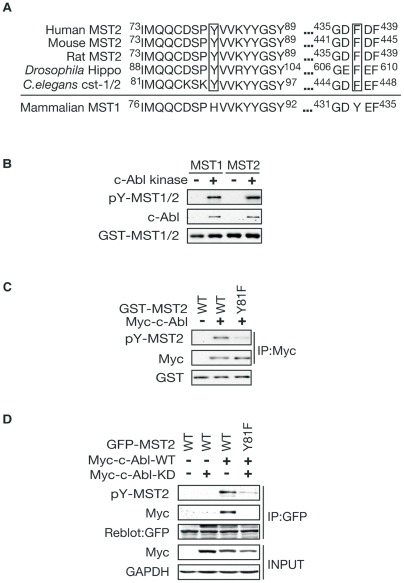
Figure 1. c-Abl Phosphorylates MST2 at Y81 in vitro and in vivo.
(A). Sequence alignment of the mammalian MST2, Drosophila Hippo, C. elegans cst-1/2 and mammalian MST1. (B). Lysates of HEK 293T cells transfected with Myc-tagged c-Abl or the control vector were immunoprecipitated with anti-Myc antibody and subjected to an in vitro kinase assay using full-length GST-MST1 or–MST2 as substrate. Phosphorylation reactions were analyzed by immunoblotting with anti-pan-tyrosine phosphorylation antibody. MST2 and MST1 proteins were tyrosine phosphorylated by c-Abl kinase in vitro. (C). In vitro kinase assay using the recombinant full-length GST-MST2-WT or–Y81F as a substrate was performed and analyzed as in A. c-Abl phosphorylated MST2 at Y81 in vitro. (D). Lysates of HEK 293T cells transfected with FLAG-MST1-WT,–Y81F expression plasmid together with Myc-c-Abl were immunoprecipitated with anti-FLAG antibody and analyzed as in A. Y81 is the phosphorylation site of MST2 by c-Abl in vivo.