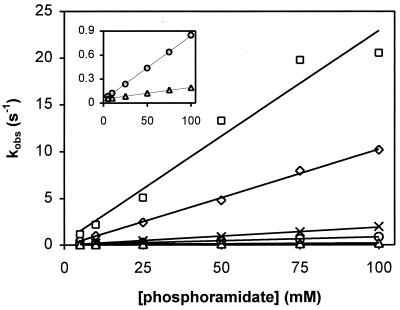
Figure 1.
Phosphorylation kinetics of wild-type CheY by PAM. Reactions were performed in the absence of peptide (○), the presence of 1 mM FliM peptide (□), the presence of 1 mM CheZ peptide (⋄), the presence of 15 μM CheA-P2 (▵), or the presence of 1 mM CheZ205VE peptide (×). Measurements were done with a stopped-flow instrument, and constant ionic strength was maintained. Observed first-order rate constants (kobs) were determined from individual phosphorylation time courses at the indicated phosphodonor concentrations. For clarity, kobs values obtained in the absence of peptide (○) and in the presence of CheA-P2 (▵) are replotted in Inset with a different y-axis scale.