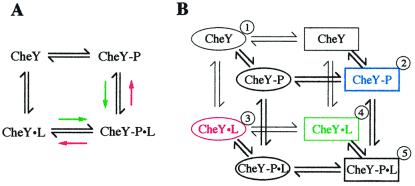
Figure 4.
Models of CheY activation. (A) Four-state model. The model describes a thermodynamic box of equilibria for coupling of the binding of a ligand to the phosphorylation of CheY. “P” denotes a phosphoryl group and “L” denotes a peptide ligand. Colored arrows indicate a shift of the respective equilibria in the presence of FliM or CheZ peptide (green) or CheA-P2 (red). See the text for further explanation. (B) Eight-state model. The described four-state model is expanded by equilibria that couple phosphorylation and ligand binding to a protein conformational change. Ellipses denote an inactive, and rectangles denote an active conformation. Equilibria are thought to be distributed as follows. ① is favored in the absence of phosphorylation and ligand. Phosphorylation stabilizes ② (blue). Binding of CheA-P2 stabilizes ③ (red), whereas binding of FliM or CheZ peptide stabilizes ④ (green). Already in active conformations, ② binds CheZ or FliM peptide with increased affinity, and ④, with CheZ or FliM peptide as ligands, phosphorylates rapidly to favor ➎. On the other hand, ② is in an unfavorable conformation to bind CheA-P2, and ③, with CheA-P2 as ligand, is in an unfavorable conformation to autophosphorylate.