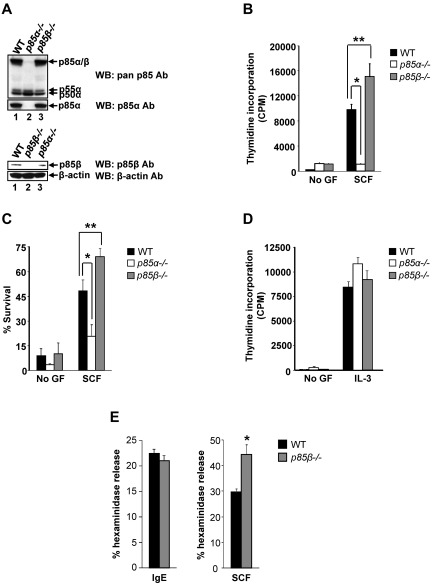
Figure 1.
Deficiency of p85β regulatory subunit of class IA PI3K results in enhanced BMMC growth and survival. (A) Expression of class IA PI3K regulatory subunits in WT, P85α−/−, and P85β−/− BMMCs. BMMCs were harvested and equal amount of protein extracts were subjected to Western blot analysis using a pan-anti–p85 antibody (this antibody recognizes all regulatory subunits of class IA PI3K), p85α-specific antibody, p85β-specific antibody, and β-actin antibody as indicated. Expression of different regulatory subunits is indicated. (B) PI3K regulatory subunits p85α and p85β differentially regulate proliferation of BMMCs. BMMCs from WT, P85α−/−, and P85β−/− mice were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, proliferation was evaluated by [3H]thymidine incorporation. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. Similar results were observed in 6 independent experiments. *P < .01, WT versus P85α−/−.**P < .01, WT versus P85β−/−. (C) Enhanced survival of p85β-deficient BMMCs. BMMCs from WT, P85α−/−, and P85β−/− mice were starved for 6 hours in serum- and cytokine-free media and cultured in the presence or absence of SCF (50 ng/mL). After 48 hours, cells were stained with PE-conjugated annexin V and 7-AAD followed by flow cytometric analysis. Shown is a representative bar graph demonstrating percentage of annexin V and 7-AAD–negative cells in the presence and absence of SCF. Similar results were observed in 4 independent experiments. *P < .05, WT versus P85α−/−. **P < .01, WT versus P85β−/−. (D) Cells described in panel B were subjected to proliferation in the presence of IL-3. Bars represent the mean [3H]thymidine incorporation in BMMCs (CPM + SD) from one representative experiment performed in quadruplicate. (E) p85β deficiency enhances SCF-mediated mast cell granule mediator release. Cells described in panel B were starved of growth factor for 6 hours and washed. Cells were sensitized with anti–DNP IgE and stimulated with DNP-BSA for 30 minutes (left panel) or cells were stimulated with SCF for 30 minutes (right panel). Degranulation was measured as β-hexosaminidase release. Similar results were observed in 2 independent experiments. *P < .05, WT versus P85β−/−.