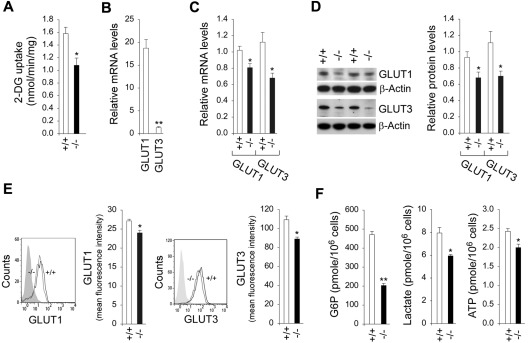
Figure 3.
Analysis of 2-DG uptake, the expression of GLUTs, and intracellular G6P, lactate, and ATP levels in G6pc3−/− macrophages. The CD11b-enriched macrophages used for function analysis were isolated from 6- to 8-week-old wild-type (+/+) and G6pc3−/− (−/−) littermates. For 2-DG uptake and quantitative real-time RT-PCR, the data represent the mean ± SEM of 4 independent experiments. (A) Uptake of 2-DG in macrophages. (B) Quantification of GLUT1 and GLUT3 mRNA in wild-type macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to 1 wild-type GLUT3 arbitrarily defined as 1. (C) Quantification of GLUT1 and GLUT3 mRNA in wild-type and G6pc3−/− macrophages by real-time RT-PCR. Expression is normalized to β-actin and measured relative to 1 wild-type mouse arbitrarily defined as 1. (D) Western blot analysis of protein extracts of peritoneal macrophages with the use of Abs against GLUT1, GLUT3, or β-actin. Each lane contains 50 μg of protein. The relative GLUT1 and GLUT3 protein levels were quantified by densitometry of 4 separate pairs of Western blots, and the measurements are relative to β-actin. (E) Quantitative flow cytometric analysis of membrane-bound GLUT1 and GLUT3 in macrophages. The gray tracing represents the fluorescence background. Data represent the mean ± SEM of 4 independent experiments. (E) Macrophage G6P, lactate, and ATP levels. Data represent the mean ± SEM of 4 independent experiments. **P < .005 and *P < .05.