Abstract
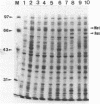
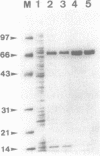
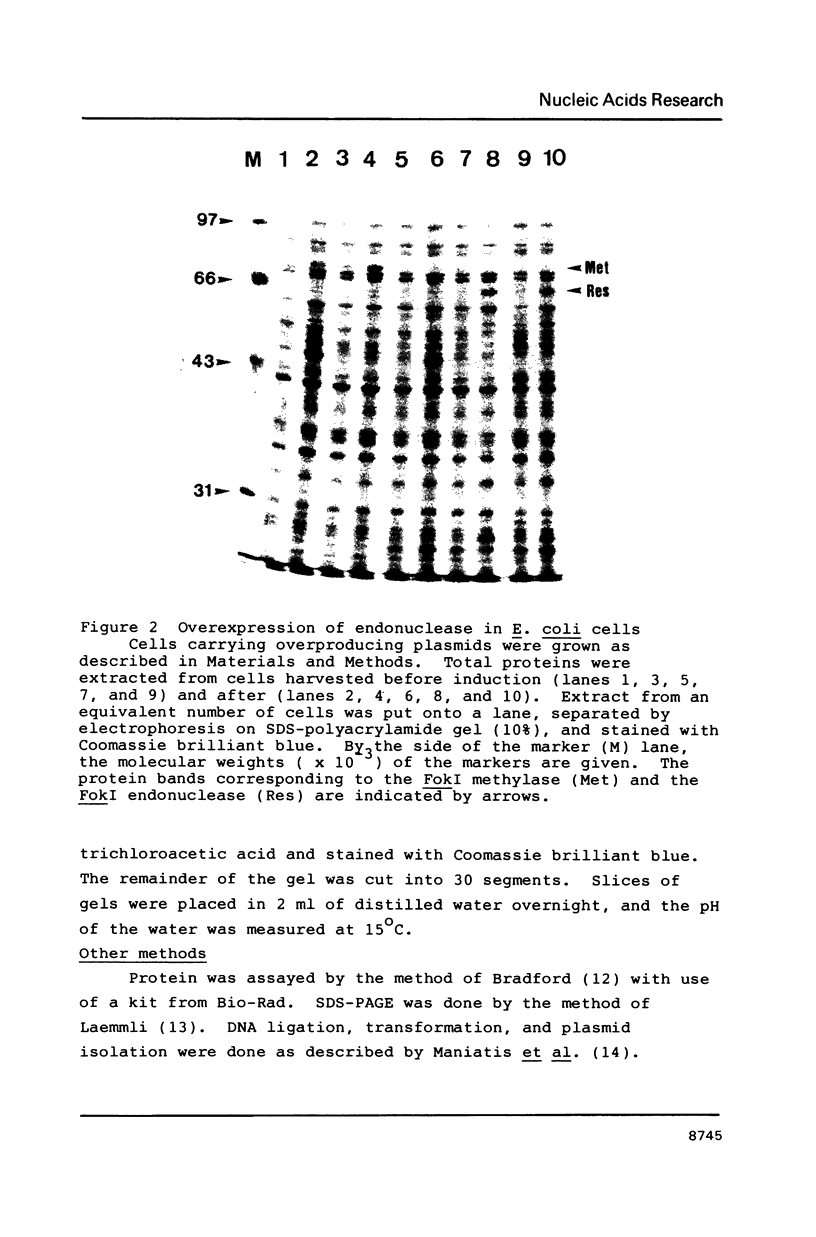
To overproduce FokI endonuclease (R.FokI) in an Escherichia coli system, the coding region of R.FokI predicted from the nucleotide sequence was generated from the FokI operon and joined to the tac promoter of an expression vector, pKK223-3. By introduction of the plasmid into E. coli UT481 cells expressing the FokI methylase gene, the R.FokI activity was overproduced about 30-fold, from which R.FokI was purified in amounts sufficient for crystallization. The removal of a stem-loop structure immediately upstream of the R.FokI coding region was essential for overproduction.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken C., Gumport R. I. Restriction endonuclease RsrI from Rhodobacter sphaeroides, an isoschizomer of EcoRI: purification and properties. Nucleic Acids Res. 1988 Aug 25;16(16):7901–7916. doi: 10.1093/nar/16.16.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany F. Overproduction, purification and crystallization of TaqI restriction endonuclease. Gene. 1988 May 30;65(2):167–177. doi: 10.1016/0378-1119(88)90453-2. [DOI] [PubMed] [Google Scholar]
- Botterman J., Zabeau M. High-level production of the EcoRI endonuclease under the control of the pL promoter of bacteriophage lambda. Gene. 1985;37(1-3):229–239. doi: 10.1016/0378-1119(85)90277-x. [DOI] [PubMed] [Google Scholar]
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougueleret L., Tenchini M. L., Botterman J., Zabeau M. Overproduction of the EcoR V endonuclease and methylase. Nucleic Acids Res. 1985 Jun 11;13(11):3823–3839. doi: 10.1093/nar/13.11.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chandrasegaran S., Smith H. O., Amzel M. L., Ysern X. Preliminary X-ray diffraction analysis of HhaII endonuclease-DNA cocrystals. Proteins. 1986 Nov;1(3):263–266. doi: 10.1002/prot.340010309. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., Kim R., King K., Kim S. H., Modrich P. Isolation of gram quantities of EcoRI restriction and modification enzymes from an overproducing strain. J Biol Chem. 1984 Sep 25;259(18):11571–11575. [PubMed] [Google Scholar]
- D'Arcy A., Brown R. S., Zabeau M., van Resandt R. W., Winkler F. K. Purification and crystallization of the EcoRV restriction endonuclease. J Biol Chem. 1985 Feb 25;260(4):1987–1990. [PubMed] [Google Scholar]
- Ishino Y., Shinagawa H., Makino K., Tsunasawa S., Sakiyama F., Nakata A. Nucleotide sequence of the lig gene and primary structure of DNA ligase of Escherichia coli. Mol Gen Genet. 1986 Jul;204(1):1–7. doi: 10.1007/BF00330179. [DOI] [PubMed] [Google Scholar]
- Kelly S., Kaddurah-Daouk R., Smith H. O. Purification of the HhaII restriction endonuclease from an overproducer Escherichia coli clone. J Biol Chem. 1985 Dec 5;260(28):15339–15344. [PubMed] [Google Scholar]
- Kim S. C., Podhajska A. J., Szybalski W. Cleaving DNA at any predetermined site with adapter-primers and class-IIS restriction enzymes. Science. 1988 Apr 22;240(4851):504–506. doi: 10.1126/science.2833816. [DOI] [PubMed] [Google Scholar]
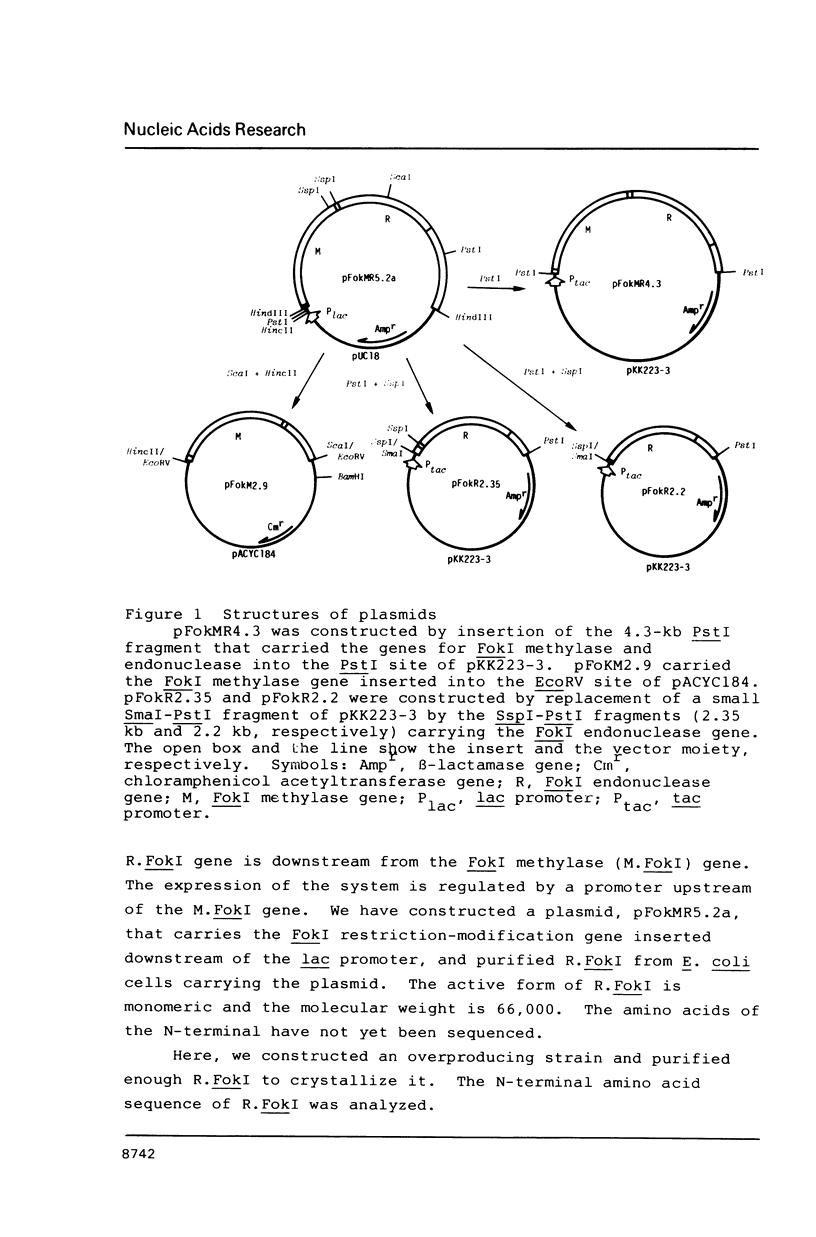
- Kita K., Kotani H., Sugisaki H., Takanami M. The fokI restriction-modification system. I. Organization and nucleotide sequences of the restriction and modification genes. J Biol Chem. 1989 Apr 5;264(10):5751–5756. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Rose R. E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988 Jan 11;16(1):355–355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. A., Modrich P. EcoRI methylase. Physical and catalytic properties of the homogeneous enzyme. J Biol Chem. 1977 Oct 25;252(20):7265–7272. [PubMed] [Google Scholar]
- Sugisaki H., Kanazawa S. New restriction endonucleases from Flavobacterium okeanokoites (FokI) and Micrococcus luteus (MluI). Gene. 1981 Dec;16(1-3):73–78. doi: 10.1016/0378-1119(81)90062-7. [DOI] [PubMed] [Google Scholar]
- Szybalski W. Universal restriction endonucleases: designing novel cleavage specificities by combining adapter oligodeoxynucleotide and enzyme moieties. Gene. 1985;40(2-3):169–173. doi: 10.1016/0378-1119(85)90039-3. [DOI] [PubMed] [Google Scholar]
- Tsunasawa S., Stewart J. W., Sherman F. Amino-terminal processing of mutant forms of yeast iso-1-cytochrome c. The specificities of methionine aminopeptidase and acetyltransferase. J Biol Chem. 1985 May 10;260(9):5382–5391. [PubMed] [Google Scholar]
- Vermersch P. S., Bennett G. N. The use of a selectable FokI cassette in DNA replacement mutagenesis of the R388 dihydrofolate reductase gene. Gene. 1987;54(2-3):229–238. doi: 10.1016/0378-1119(87)90491-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]