Abstract
Background
The current study was carried out to determine whether fasudil hydrochloride (fasudil), a Rho-kinase inhibitor, has myocardial postconditioning (PostC) activity under hyperglycemia as well as normoglycemia, and if so, whether the effects could be mediated by mitochondrial ATP-sensitive potassium (m-KATP) channels.
Methods
Male Sprague-Dawley rats were anesthetized with sodium pentobarbital. After opening the chest, all rats underwent 30-min coronary artery occlusion followed by 2-h reperfusion. The rats received low-dose (0.15 mg/kg) or high-dose (0.5 mg/kg) fasudil or diazoxide, an m-KATP channel opener, at 10 mg/kg, just before reperfusion under normoglycemic or hyperglycemic conditions. In another group, rats received 5-hydroxydecanoic acid (5HD), an m-KATP channel blocker, at 10 mg/kg, before high-dose fasudil. Myocardial infarct size was expressed as a percentage of area at risk (AAR).
Results
Under normoglycemia, low-dose and high-dose fasudil and diazoxide reduced myocardial infarct size (23 ± 8%, 21 ± 9% and 21 ± 10% of AAR, respectively) compared with that in the control (42 ± 7%). Under hyperglycemia, low-dose fasudil (40 ± 11%) and diazoxide (44 ± 14%) could not exert this beneficial effect, but high-dose fasudil reduced myocardial infarct size in the same manner as under normoglycemia (21 ± 13%). 5HD prevented fasudil-induced reduction of myocardial infarct size (42 ± 13%).
Conclusion
Fasudil induces PostC against myocardial infarction via activation of m-KATP channels in the rat. Although hyperglycemia attenuates the PostC, high-dose fasudil can restore cardioprotection.
Keywords: Hyperglycemia, Postconditioning, Myocardial Infarction, Fasudil, Mitochondrial KATP channels
Introduction
Rho-kinase, a ubiquitously expressed serine-threonine protein kinase that is involved in diverse cellular functions, may play a pivotal role in cardiovascular diseases such as vasospastic angina, ischemic stroke and heart failure [1]. Some reports showed that fasudil hydrochloride (fasudil), a Rho-kinase inhibitor, had beneficial effects in ischemic heart disease. A double-blind placebo-controlled trial showed that fasudil increased the ischemic threshold of angina patients during exercise with a trend toward increased exercise duration [2]. Intracoronary administration of fasudil increased oxygen saturation in coronary sinus vein, and ameliorated pacing-induced myocardial ischemia in patients with effort angina [3]. Rho-kinase was activated in ischemic myocardium, and a Rho-kinase inhibitor lead to the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/eNOS signaling pathway, resulting in preconditioning (PreC) or postconditioning (PostC) [4,5]. Furthermore, hydroxyfasudil was also reported to activate ATP-sensitive potassium (KATP) channels via inhibition of Rho-kinase during myocardial remote peri-conditioning [6]. However, it is not clear whether mitochondrial KATP (m-KATP) channels mediate the Rho-kinase inhibitor-induced PostC.
Hyperglycemia is an important predictor and a risk factor of cardiovascular morbidity and mortality in patients with and without diabetes mellitus [7-9]. A number of studies have shown that the cardioprotective effects of pharmacological PreC and PostC are impaired by hyperglycemia [10-16]. Although the detailed mechanisms are unclear, it has been suggested that the loss of cardioprotection by hyperglycemia might be associated with several mechanisms, namely, generation of large quantities of reactive oxygen species (ROS), inhibition of protective signaling mechanisms, down-regulation of endothelial nitric synthase (eNOS), reduced activation and availability of protective molecules, coronary vascular endothelial dysfunction, and/or impairment in the function of m-KATP channels [11-13,17,18]. However, the effect of hyperglycemia on the Rho-kinase inhibitor-induced myocardial protection against ischemic reperfusion injury is unknown.
The present study was carried out to clarify whether fasudil has PostC activity and, if so, whether the cardioprotective effect is mediated by m-KATP channels and attenuated by hyperglycemia.
Materials and methods
All experimental procedures and protocols described in this study were approved by the Institutional Animal Care and Use Committee of Nagasaki University School of Medicine.
Drugs
Fasudil was purchased from Asahikasei Pharma (Tokyo, Japan). 5-hydroxydecanoic acid (5-HD), an m-KATP channel blocker, diazoxide, an m-KATP channel opener, patent blue dye, and 2,3,5-triphenyltetrazolium chloride (TTC) were purchased from Sigma (St. Louis, MO, USA).
General preparation
The instrumental methods used were as described in our previous report [19]. Male Sprague-Dawley (SD) rats weighing between 350 and 550 g were anesthetized with sodium pentobarbital (a 50 mg/kg intraperitoneal bolus followed by an intravenous infusion of 10-20 mg/kg/h). The rats were adequately sedated to ensure that pedal and palpebral reflexes were absent throughout the experimental protocol. Catheters were inserted into the right jugular vein for fluid or drug administration and the right carotid artery for measurement of arterial blood pressure. After tracheotomy had been performed, the trachea was intubated with a cannula connected to a small animal ventilator (SAR-830 CWE, PA, USA), and the lungs were ventilated with pure oxygen. Arterial blood gas pH was maintained within a physiological range by adjusting the respiratory rate and tidal volume throughout the experiment. A left thoracotomy was performed in the fifth intercostal space, and the pericardium was opened. A 7-0 prolene ligature was placed around the proximal left anterior descending coronary artery (LAD) and vein in the area immediately below the left atrial appendage. The ends of the suture were threaded through a small plastic tube to form a snare for reversible LAD occlusion. Coronary artery occlusion was produced by clamping the snare onto the epicardial surface of the heart and was confirmed by the appearance of epicardial cyanosis. Reperfusion was achieved by loosening the snare and was verified by observing an epicardial hyperemic response. Hemodynamics were continuously monitored with a transducer (sck-9082, Becton Dickinson, Tokyo, Japan) and a blood pressure amplifier (AP-641 G, Nihon-Kohden, Tokyo, Japan) and shown on a polygraph system (RMP-6018, Nihon-Kohden, Tokyo, Japan).
Experimental protocol
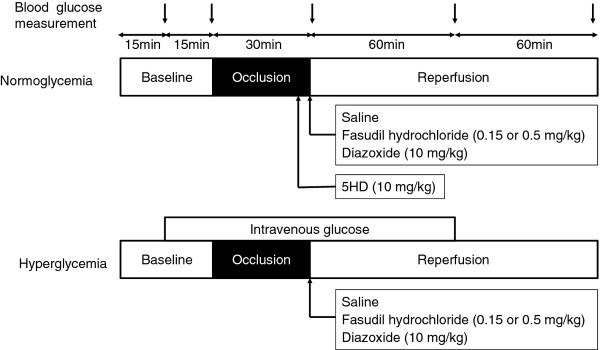
The classification of experimental groups is shown in Table 1, and the experimental design is illustrated in Figure 1. All rats underwent 30-min LAD occlusion followed by 2-h reperfusion. The rats were randomly assigned to one of 10 groups (n = 10 for each). Six of them were under normoglycemic conditions, and 4 of them were under hyperglycemic conditions. The hyperglycemia was produced with continuous infusion of 50% glucose in water starting at 15 min before LAD occlusion and lasting until 60 min after reperfusion. The target hyperglycemia was 350 mg/dl.
Table 1.
The classification of experimental groups
| Groups | Treatments |
|---|---|
| CON | Saline, i.v. bolus just before reperfusion. |
| LF | Low-dose fasudil (0.15 mg/kg), i.v. bolus just before reperfusion. |
| HF | High-dose fasudil (0.5 mg/kg), i.v. bolus just before reperfusion. |
| DIA | Diazoxide (10 mg/kg), i.v. bolus just before reperfusion. |
| HG | Saline, i.v. bolus just before reperfusion, and continuous infusion of 50% glucose in water starting at 15 min before LAD occlusion and lasting until 60 min after reperfusion. The target blood glucose concentration was 350 mg/dl. |
| LF + HG | Same as group HG except for low-dose fasudil (0.15 mg/kg) in place of saline. |
| HF + HG | Same as group HG except for low-dose fasudil (0.5 mg/kg) in place of saline. |
| DIA + HG | Same as group HG except for diazoxide (10 mg/kg) in place of saline. |
| 5HD | 5HD (10 mg/kg), i.v. at 5 min before reperfusion followed by saline, i.v. bolus just before reperfusion. |
| HF + 5HD | Same as group 5HD except for high-dose fasudil (0.5 mg/kg) in place of saline. |
Figure 1.
A schematic illustration of the experimental protocols.
In 4 normoglycemic groups, rats received saline as control (group CON), low-dose fasudil (0.15 mg/kg; group LF), high-dose fasudil (0.5 mg/kg; group HF) or diazoxide (10 mg/kg; group DIA) by i.v. bolus just before reperfusion.
In 2 normoglycemic groups, rats received 5HD, at 10 mg/kg, by i.v. bolus at 5 min before reperfusion followed by saline (group 5HD) or high-dose fasudil (group HF + 5HD) by i.v. bolus just before reperfusion.
In 4 hyperglycemic groups, rats received saline (group HG), low-dose fasudil (group LF + HG), high-dose fasudil (group HF + HG) or daizoxide (group DIA + HG).
The dose of fasudil was set on the basis of the clinical dosage for cerebral vasospasm after subarachnoid hemorrhage [20] as determined in our preliminary study. The dose of diazoxide was set on the basis of our preliminary study, which showed that 10 mg/kg but not 3 mg/kg diazoxide reduces infarct size (data not shown). The dose of 5HD was set on the basis of a previous study [19].
Blood glucose measurement
Blood samples were collected to measure blood glucose in each group. The blood glucose concentration was determined by the glucose oxidase method using a blood glucose meter (Glutest Ace, Sanwa Kagaku Kenkyusho, Nagoya, Japan). Samples were taken before starting glucose infusion, just before ischemia, just after starting reperfusion, and at 1 and 2 h after starting reperfusion.
Determination of infarct size
Myocardial infarct size was measured as previously described [21]. Briefly, at the end of each experiment, the LAD was reoccluded, and patent blue dye was administered intravenously to stain the normal region of the left ventricle (LV), and the heart was rapidly excised. LV tissue was isolated and cut into approximately ten cross-sectional pieces of equal thickness. The nonstained LV area at risk (AAR) was separated from surrounding blue-stained LV normal zone, and both regions were separately incubated at 37°C for 15 min in 1% TTC in 0.1 M phosphate buffer adjusted to pH 7.4. The tissues were fixed overnight in 10% formaldehyde. AAR and blue-stained LV normal zone region were weighed for determination of AAR/LV. TTC stains living tissue a deep red color, but necrotic tissue is TTC-negative and appears white within the AAR slices. Each slice was scanned at 1,200 dpi with a commercial scanner (Canoscan LiDE 60; Canon, Japan), and infarcted and noninfarcted areas were measured using an image analysis program. Myocardial infarct size is expressed as a percentage of the AAR.
Statistical analysis
Statistical analyses of hemodynamic data and blood glucose concentrations within and between groups were performed with analysis of variance for repeated measures followed by Dunnett's test. Inter-group differences in body weight, age, LV weight, AAR weight, the ratio of AAR to LV and the ratio of infarct size to AAR were analyzed using one-way analysis of variance followed by the Student-Newman-Keuls test. Statistical significance was defined as P < 0.05. All values are expressed as mean ± SD. Statistical analysis was performed using SPSS 15.0 software (SPSS Japan, Tokyo, Japan) or GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
Results
Hemodynamics
There were no significant differences in body weight or age among the groups. Hemodynamic data for heart rate (HR) and mean blood pressure (MBP) are shown in Table 2. There were no significant differences either within groups or among groups in HR or MBP at any measurement point.
Table 2.
Systemic hemodynamic
| Baseline | Occlusion 20 min | Reperfusion 20 min | 1 h after reperfusion | 2 h after reperfusion | ||
|---|---|---|---|---|---|---|
| CON | HR | 112 ± 15 | 114 ± 20 | 120 ± 13 | 109 ± 15 | 101 ± 14 |
| MAP | 423 ± 30 | 420 ± 34 | 425 ± 36 | 410 ± 38 | 407 ± 35 | |
| LF | HR | 114 ± 12 | 119 ± 12 | 113 ± 14 | 104 ± 9 | 96 ± 9 |
| MAP | 431 ± 28 | 427 ± 30 | 415 ± 34 | 405 ± 35 | 393 ± 35 | |
| HF | HR | 107 ± 30 | 111 ± 28 | 106 ± 26 | 114 ± 25 | 115 ± 18 |
| MAP | 408 ± 37 | 381 ± 57 | 395 ± 38 | 404 ± 36 | 392 ± 52 | |
| DIA | HR | 104 ± 16 | 100 ± 20 | 97 ± 21 | 106 ± 28 | 106 ± 28 |
| MAP | 403 ± 62 | 399 ± 66 | 388 ± 79 | 393 ± 69 | 400 ± 61 | |
| 5HD | HR | 104 ± 16 | 100 ± 20 | 102 ± 18 | 114 ± 25 | 110 ± 24 |
| MAP | 394 ± 44 | 384 ± 52 | 385 ± 50 | 370 ± 32 | 407 ± 53 | |
| HF + 5HD | HR | 108 ± 24 | 107 ± 27 | 112 ± 28 | 95 ± 27 | 99 ± 20 |
| MAP | 404 ± 69 | 392 ± 51 | 392 ± 60 | 384 ± 69 | 397 ± 47 | |
| HG | HR | 107 ± 20 | 91 ± 15 | 116 ± 25 | 113 ± 19 | 104 ± 15 |
| MAP | 409 ± 33 | 403 ± 34 | 409 ± 36 | 396 ± 30 | 397 ± 40 | |
| LF + HG | HR | 101 ± 12 | 93 ± 16 | 96 ± 19 | 106 ± 20 | 110 ± 17 |
| MAP | 406 ± 42 | 388 ± 58 | 386 ± 60 | 398 ± 55 | 408 ± 53 | |
| HF + HG | HR | 100 ± 29 | 104 ± 27 | 115 ± 25 | 108 ± 24 | 89 ± 30 |
| MAP | 407 ± 65 | 407 ± 28 | 383 ± 64 | 376 ± 64 | 380 ± 76 | |
| DIA + HG | HR | 106 ± 24 | 104 ± 16 | 102 ± 21 | 97 ± 18 | 104 ± 23 |
| MAP | 389 ± 48 | 398 ± 53 | 396 ± 30 | 389 ± 45 | 383 ± 58 |
Data are mean ± SD, and n = 10 for each.
HR = heart rate (beats/min); MAP = mean arterial pressure (mmHg); CON = control; LF or HR = 0.15 or 0.5 mg/kg fasudil hydrochloride, DIA = 10 mg/kg diazoxide; 5HD = 5-hydroxydecanoic acid; HG = hyperglycemia.
Infarct size
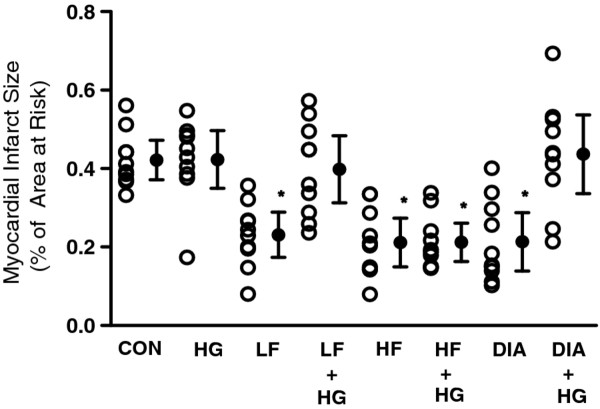
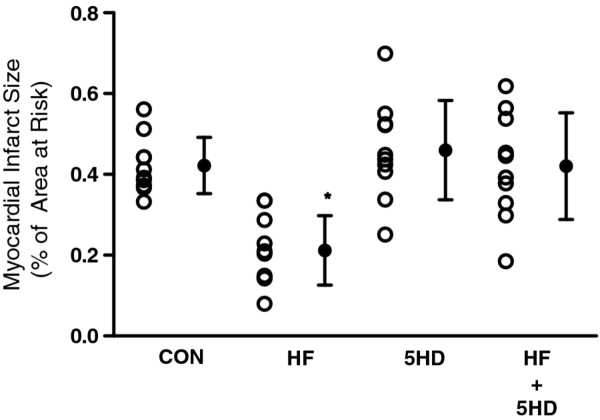
There were no significant differences in LV weight, AAR weight or the ratio of AAR to total LV mass among the groups (Table 3). Infarct sizes expressed as a percentage of AAR are shown in Figures 2, 3. In groups LF, HF and DIA, the infarct sizes were significantly reduced compared with that in group CON (Figure 2). In groups LF + HG and DIA + HG, the infarct sizes were similar to that in group CON (Figure 2). In contrast, the infarct size in group HF + HG was significantly less than that in group CON. In group HF + 5HD, the infarct size was similar to that in group CON (Figure 3).
Table 3.
Left ventricular area at risk and infarct size
| AAR/LV (%) | IS/AAR (%) | |
|---|---|---|
| CON | 51 ± 9 | 42 ± 7 |
| LF | 46 ± 9 | 23 ± 8* |
| HF | 52 ± 9 | 21 ± 9* |
| DIA | 46 ± 7 | 21 ± 10* |
| 5HD | 48 ± 8 | 46 ± 12 |
| HF + 5HG | 44 ± 7 | 42 ± 13 |
| HG | 43 ± 13 | 42 ± 10 |
| LF + HG | 50 ± 9 | 40 ± 11 |
| HF + HG | 45 ± 7 | 21 ± 13* |
| DIA + HG | 48 ± 9 | 44 ± 14 |
Date are mean ± SD, and n = 10 for earch.
CON = control; LF or HF = 0.15 or 0.5 mg/kg fasudil hydrochloride; DIA = 10 mg/kg diazoxide; 5HD = 5-hydroxydecanoic acid; HG = hyperglycemia.
*Significantly (p < 0.05) different from control.
Figure 2.
Myocardial infarct size expressed as a percentage of the left ventricular area at risk. Hyperglycemia (HG) group was administered 50% glucose during occlusion and until 1 h after reperfusion. CON received saline just before reperfusion; LF or HF, 0.15 or 0.5 mg/kg fasudil hydrochloride; and DIA, 10 mg/kg diazoxide. Data are mean ± SD and n = 10 for each. *Significantly (p < 0.05) different from control.
Figure 3.
Myocardial infarct size expressed as a percentage of the left ventricular area at risk. 5HD group was administered 10 mg/kg 5-hydroxydecanoic acid at 5 min before reperfusion. CON received saline just before reperfusion; HF, 0.5 mg/kg fasudil hydrochloride. *Significantly (p < 0.05) different from control.
Blood glucose concentrations
As shown in Table 4, blood glucose concentrations at baseline did not differ among the groups. Blood glucose concentrations did not change significantly over time in normoglycemic groups. In hyperglycemic groups, blood glucose concentrations at just before ischemia, just after starting reperfusion and 1 h after starting reperfusion were significantly higher than those at baseline and 2 h after reperfusion. There were no significant differences in blood glucose concentrations among the hyperglycemic groups at any measured point.
Table 4.
Blood glucose concentration (mg/dl)
| Baseline | Before ischemia | Just after reperfusion | 1 h after reperfusion | 2 h after reperfusion | |
|---|---|---|---|---|---|
| CON | 88 ± 15 | 91 ± 19 | 112 ± 27 | 105 ± 32 | 101 ± 20 |
| LF | 95 ± 11 | 109 ± 14 | 122 ± 26 | 118 ± 26 | 100 ± 23 |
| HF | 89 ± 17 | 87 ± 13 | 111 ± 33 | 93 ± 35 | 95 ± 26 |
| DIA | 90 ± 14 | 88 ± 14 | 107 + 31 | 120 ± 35 | 107 ± 28 |
| 5HD | 89 ± 12 | 85 ± 16 | 101 ± 28 | 108 ± 18 | 111 ± 28 |
| HF + 5HD | 87 ± 8 | 86 ± 9 | 105 ± 24 | 101 ± 26 | 104 ± 24 |
| HG | 92 ± 12 | 349 ± 32*† | 360 ± 50*† | 353 ± 28*† | 100 ± 39 |
| LF + HG | 92 ± 13 | 338 ± 24*† | 378 ± 57*† | 327 ± 29*† | 105 ± 42 |
| HF + HG | 92 ± 12 | 341 ± 25*† | 374 ± 52*† | 337 ± 47*† | 110 ± 28 |
| DIA + HG | 91 ± 13 | 349 ± 22*† | 350 ± 53*† | 353 ± 28*† | 106 ± 37 |
Data are mean ± SD, and n = 10 for each.
CON = control; LF or HF = 0.15 or 0.5 mg/kg fasudil hydrochloride; DIA = 10 mg/kg diazoxide; 5HD = 5-hydroxydecanoic acid; HG = hyperglycemia.
*Significantly (p < 0.05) different from baseline.
†Significantly (p < 0.05) different from control.
Discussion
The present results show that fasudil induces postconditioning against myocardial infarction via m-KATP channels in the rat, and that hyperglycemia prevents fasudil- and diazoxide-induced postconditioning, whereas high-dose fasudil can restore cardioprotection even under hyperglycemia.
In the present results, both high and low doses of fasudil produced equivalent reductions of myocardial infarct size under normoglycemia. However, hyperglycemia completely abolished the protective effect of the low dose, but not the high dose of fasudil. Fasudil is clinically used in doses of 30 mg/30 min intravenous infusion three times a day for cerebral vasospasm after subarachnoid hemorrhage [20], and 60 mg/60 min intravenous infusion twice daily for acute ischemic stroke [22]. Thus, the high dose used in the present study is relevant to the clinical dose, and it is indicated that fasudil at a clinical dose could induce PostC even under hyperglycemia.
Kersten et al. [12] showed that PreC evoked by 2.5 mg/kg but not 5 mg/kg diazoxide was blocked by hyperglycemia, and they assumed that the blockage of m-KATP channels was the underlying mechanism of the lack of myocardial protection by volatile anesthetics under acute hyperglycemia. Recently our group reported that hyperglycemia raised the threshold of levosimendan-induced PostC [23], and it is suggested that levosimendan-induced PostC involves the activation of m-KATP channels [24]. In the present results, low-dose fasudil- and diazoxide-induced PostC were attenuated by hyperglycemia, and 5-HD inhibited the fasudil-induced PostC. Therefore, it is likely that fasudil-induced PostC is mediated by m-KATP channels, which could be attenuated by hyperglycemia.
Zaho et al. [6] reported that the peri-conditioning stimulus of hydroxyfasudil was abrogated by glibenclamide, a non-selective KATP channel blocker, and suggested that activation of KATP channels could be mediated by Rho-kinase inhibition. Although some studies on PostC that targeted KATP channels generated negative results [25,26], a number of studies have revealed an obligatory role of m-KATP channels in both ischemic and pharmacological PostC [27-31]. Wolfrum et al. [4] reported that the Rho-kinase inhibition led to the activation of PI3K/Akt/eNOS pathway and PreC. Hamid et al. [5] showed that inhibition of Rho-kinase by Y27632, a structurally unrelated inhibitor of hydroxyfasudil, at reperfusion onset limited infarct size through an Akt/eNOS-dependent mechanism. Consequently, it is suggested that Rho-kinase acts as a negative regulator of PI3K activation, and modulates the protective effect via PI3K/Akt/eNOS pathway on myocardial PreC and PostC. Some kinds of pharmacological PostC were shown to depend on PI3K/Akt/eNOS pathway with a target downstream of m-KATP channels [27,28]. Therefore, it is likely that fasudil could induce the opening of m-KATP channels by up-regulating PI3K/Akt/eNOS pathway, resulting in induction of PostC.
Rho kinase activity is involved in diabetic endothelial dysfunction, and regulation of Rho kinase signaling is important for cellular function, such as contraction, mortality, proliferation, apoptosis [32]. Correspondingly, Rho-kinase is known to be activated in ischemic myocardium, and inhibition of Rho-kinase activity is a matter of great interest on myocardial protection against ischemic reperfusion injury [33]. However, there are few reports on the effect of hyperglycemia on the myocardial Rho-kinase activity during ischemia-reperfusion. It was shown that exposure of endothelial cells, renal glomerulus and cardiac fibroblasts to hyperglycemia increases Rho-kinase activity [34-37], and that the Rho-kinase activity occurred in a glycemic concentration-dependent manner [34,37]. Furthermore, the protective effect of Rho-kinase inhibitor depends on the dose under hyperglycemia [34,35,37]. Thus, it is likely that hyperglycemia could increase the myocardial Rho-kinase activity during ischemia-reperfusion, and raise the threshold of fasudil-induced PostC.
Hyperglycemia is an important predictor and a risk factor of cardiovascular morbidity and mortality [7-9], and pharmacological cardioprotection against ischemia-reperfusion injury is impaired by hyperglycemia, independent of diabetes [10-16]. PostC is considered to be more feasible than PreC for clinical application, and would be useful in unpredictable myocardial ischemia-reperfusion injury. Nevertheless, there are only a few reports concerning pharmacological PostC under hyperglycemia. Raphael et al. [11] showed that hyperglycemia inhibited PostC provided by isoflurane, and that the effect was mediated via modulation of PI3K/Akt/eNOS signaling. Huhn et al. [10] reported that sevoflurane-induced PostC was blocked by hyperglycemia, but the inhibition of mitochondrial permeability transition pore with cyclosporine A could reverse this loss of cardioprotection. Therefore, the present report is the first describe about Rho-kinase inhibitor-induced PostC under hyperglycemia.
Przyklen et al. [38] reported that diabetic hearts were refractory to ischemic PostC, but the effect in the type 1 diabetic model was reversed by restoration of normoglycemia. Whereas, Drenger et al. [27] showed that the protective effect of ishemic and sevoflurane PostC were not relived by insulin-induced normoglycemia in diabetic rats. Tsang et al. [39] reported that it was necessary to increase the ischemic PreC stimulus to achieve the threshold for cardioprotection and a critical level of Akt phosphorylation to mediate myocardial protection in diabetes. Furthermore, rats with type 1 diabetes for 4 weeks showed ischemic PreC weaker than normal rats, but there was no cardioprotection in the rats for 8 weeks [40]. These finding suggested that the threshold for cardioprotection in diabetes will gradually increase with the prolongation of hyperglycemic time. Therefore, it is likely that the threshold of PostC in the diabetic animals or in animals exposed to longer periods of hyperglycemia is higher than acute hyperglycemia.
Marinovic et al. [41] reported that sarcolemma KATP (s-KATP) channels act as an effector and m-KATP channels play a dual role as a trigger and an effector in cardioprotection by isoflurane-induced PreC against oxidative stress. Furthermore, Yang et al. [42] showed that KR31761, a new KATP channel opener, exerted PreC in rats, and both 5-HD and HMR-1098, an s-KATP channel blocker, prevents the cardioprotective effects. Chen et al. [43] reported that s-KATP channels were decreased in diabetes and under hyperglycemia, and it lead to result in desensitization of some KATP channel drugs. Thus, there is possibility that cardiprotection via s-KATP channels is attenuated under hyperglycemia. However, mechanism for s-KATP channels dependent PreC and PostC is not clarified, and there are few reports about s-KATP channels involved with rho-kinase. Further research is needed to elucidate the molecular mechanisms contributing to this cardioprotective effect.
Conclusion
Fasudil induces PostC against myocardial infarction in the rat. This protective effect of fasudil is mediated by activation of m-KATP channels. Although hyperglycemia raises the threshold of fasudil-induced PostC, high-dose fasudil could preserve the protective effect.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TI performed experiments, contributed to discussion, and drafted the manuscript. SC participated in the design and coordination of the study, contributed to discussion and reviewed/edited the manuscript. UH and SM helped carry out in vivo experiments. TM participated in the design and coordination of the study. KS supervised research to discussion and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Contributor Information
Taiga Ichinomiya, Email: t0ra_t0ra_t0ra@hotmail.com.
Sungsam Cho, Email: chos@net.nagasaki-u.ac.jp.
Ushio Higashijima, Email: ushioh@nagasaki-u.ac.jp.
Shuhei Matsumoto, Email: shumtmt@nagasaki-u.ac.jp.
Takuji Maekawa, Email: mkw-t@nagasaki-u.ac.jp.
Koji Sumikawa, Email: sumikawa@nagasaki-u.ac.jp.
Acknowledgements
This work was supported in part by Grants-In Aid (to Dr. Ichinomiya) and (to Dr. Sumikawa) for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H. Thadani U; Fasudil Study Group: Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Mohri M, Inokuchi K, Ito A, Hirakawa Y, Masumoto A, Hirooka Y, Takeshita A, Shimokawa H. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol. 2007;49:117–121. doi: 10.1097/FJC.0b013e31802ef532. [DOI] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid SA, Bower HS, Baxter GF. Rho kinase activation plays a major role as a mediator of irreversible injury in reperfused myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2598–H2606. doi: 10.1152/ajpheart.01393.2006. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Yang YJ, Pei WD, Sun YH, You SJ, Gao RL. Remote periconditioning reduces myocardial no-reflow by the activation of K ATP channel via inhibition of Rho-kinase. Int J Cardiol. 2009;133:179–184. doi: 10.1016/j.ijcard.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Ergelen M, Uyarel H, Cicek G, Isik T, Osmonov D, Gunaydin ZY, Bozbay M, Turer A, Gul M, Abanonu GB, Ilhan E. Which is worst in patients undergoing primary angioplasty for acute myocardial infarction? Hyperglycaemia? Diabetes mellitus? Or both? Acta Cardiol. 2010;65:415–423. doi: 10.2143/AC.65.4.2053900. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- Noordzij PG, Boersma E, Schreiner F, Kertai MD, Feringa HH, Dunkelgrun M, Bax JJ, Klein J, Poldermans D. Increased preoperative glucose levels are associated with perioperative mortality in patients undergoing noncardiac, nonvascular surgery. Eur J Endocrinol. 2007;156:137–142. doi: 10.1530/eje.1.02321. [DOI] [PubMed] [Google Scholar]
- Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–471. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- Raphael J, Gozal Y, Navot N, Zuo Z. Hyperglycemia inhibits anesthetic-induced postconditioning in the rabbit heart via modulation of phosphatidylinositol-3-kinase/Akt and endothelial nitric oxide synthase signaling. J Cardiovasc Pharmacol. 2010;55:348–357. doi: 10.1097/FJC.0b013e3181d26583. [DOI] [PubMed] [Google Scholar]
- Kersten JR, Montgomery MW, Ghassemi T, Gross ER, Toller WG, Pagel PS, Warltier DC. Diabetes and hyperglycemia impair activation of mitochondrial K(ATP) channels. Am J Physiol Heart Circ Physiol. 2001;280:H1744–H1750. doi: 10.1152/ajpheart.2001.280.4.H1744. [DOI] [PubMed] [Google Scholar]
- Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology. 2002;96:183–188. doi: 10.1097/00000542-200201000-00032. [DOI] [PubMed] [Google Scholar]
- LaDisa JF Jr, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Cardioprotection by glucose-insulin-potassium: dependence on KATP channel opening and blood glucose concentration before ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H601–H607. doi: 10.1152/ajpheart.00122.2004. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim SY, Kwak YL, Hwang KC, Shim YH. Hyperglycemia Attenuates Myocardial Preconditioning of Remifentanil. J Surg Res. 2011. in press . [DOI] [PubMed]
- Wong VW, Mardini M, Cheung NW, Mihailidou AS. High-dose insulin in experimental myocardial infarction in rabbits: protection against effects of hyperglycaemia. J Diabetes Complications. 2011;25:122–128. doi: 10.1016/j.jdiacomp.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Balakumar P, Singh H, Singh M, Anand-Srivastava MB. The impairment of preconditioning-mediated cardioprotection in pathological conditions. Pharmacol Res. 2009;60:18–23. doi: 10.1016/j.phrs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Kehl F, Krolikowski JG, Weihrauch D, Pagel PS, Warltier DC, Kersten JR. N-acetylcysteine restores isoflurane-induced preconditioning against myocardial infarction during hyperglycemia. Anesthesiology. 2003;98:1384–1390. doi: 10.1097/00000542-200306000-00013. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Cho S, Tosaka S, Ureshino H, Maekawa T, Hara T, Sumikawa K. Pharmacological preconditioning in type 2 diabetic rat hearts: the roles of mitochondrial ATP-sensitive potassium channels and the phosphatidylinositol 3-kinase-Akt pathway. Cardiovasc Drugs Ther. 2009;23:263–270. doi: 10.1007/s10557-009-6184-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, Nagata I, Kikuchi H, Takemae T, Hidaka H, Nakashima M. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg. 1992;76:571–577. doi: 10.3171/jns.1992.76.4.0571. [DOI] [PubMed] [Google Scholar]
- Ludwig LM, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Protein kinase C translocation and Src protein tyrosine kinase activation mediate isoflurane-induced preconditioning in vivo: potential downstream targets of mitochondrial adenosine triphosphate-sensitive potassium channels and reactive oxygen species. Anesthesiology. 2004;100:532–539. doi: 10.1097/00000542-200403000-00011. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Hirai S, Seto M, Satoh S. Ohtomo E; Fasudil Ischemic Stroke Study Group: Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Cho S, Tosaka S, Higashijima U, Maekawa T, Hara T, Sumikawa K. Hyperglycemia raises the threshold of levosimendan- but not milrinone-induced postconditioning in rat hearts. Cardiovasc Diabetol. 2012;11:4. doi: 10.1186/1475-2840-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönisch A, Theuring N, Ebner B, Wagner C, Strasser RH, Weinbrenner C. Postconditioning with levosimendan reduces the infarct size involving the PI3K pathway and KATP-channel activation but is independent of PDE-III inhibition. Basic Res Cardiol. 2010;105:155–167. doi: 10.1007/s00395-009-0064-9. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H. Kitamura S; J-WIND investigators: Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- Imagawa J, Baxter GF, Yellon DM. Myocardial protection afforded by nicorandil and ischaemic preconditioning in a rabbit infarct model in vivo. J Cardiovasc Pharmacol. 1998;31:74–79. doi: 10.1097/00005344-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Sumi S, Kobayashi H, Yasuda S, Iwasa M, Yamaki T, Yamada Y, Ushikoshi H, Hattori A, Aoyama T, Nishigaki K, Takemura G, Minatoguchi S. Postconditioning effect of granulocyte colony-stimulating factor is mediated through activation of risk pathway and opening of the mitochondrial KATP channels. Am J Physiol Heart Circ Physiol. 2010;299:H1174–H1182. doi: 10.1152/ajpheart.00116.2010. [DOI] [PubMed] [Google Scholar]
- Hönisch A, Theuring N, Ebner B, Wagner C, Strasser RH, Weinbrenner C. Postconditioning with levosimendan reduces the infarct size involving the PI3K pathway and KATP-channel activation but is independent of PDE-III inhibition. Basic Res Cardiol. 2010;105:155–167. doi: 10.1007/s00395-009-0064-9. [DOI] [PubMed] [Google Scholar]
- Drenger B, Ostrovsky IA, Barak M, Nechemia-Arbely Y, Ziv E, Axelrod JH. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology. 2011;114:1364–1372. doi: 10.1097/ALN.0b013e31820efafd. [DOI] [PubMed] [Google Scholar]
- Obal D, Dettwiler S, Favoccia C, Scharbatke H, Preckel B, Schlack W. The influence of mitochondrial KATP-channels in the cardioprotection of preconditioning and postconditioning by sevoflurane in the rat in vivo. Anesth Analg. 2005;101:1252–1260. doi: 10.1213/01.ANE.0000181336.96511.32. [DOI] [PubMed] [Google Scholar]
- Krolikowski JG, Bienengraeber M, Weihrauch D, Warltier DC, Kersten JR, Pagel PS. Inhibition of mitochondrial permeability transition enhances isoflurane-induced cardioprotection during early reperfusion: the role of mitochondrial KATP channels. Anesth Analg. 2005;101:1590–1596. doi: 10.1213/01.ANE.0000181288.13549.28. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu H, Chen B, Li Q, Huang X, Wang L, Guo X, Huang Q. RhoA/ROCK-dependent moesin phosphorylation regulates AGE-induced endothelial cellular response. Cardiovasc Diabetol. 2012;11:7. doi: 10.1186/1475-2840-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Bian HJ, Li XX, Liu XB, Sun JP, Li N, Zhang Y, Ji XP. ERK-MAPK signaling opposes rho-kinase to reduce cardiomyocyte apoptosis in heart ischemic preconditioning. Mol Med. 2010;16:307–315. doi: 10.2119/molmed.2009.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H, Okamoto R, Kato S, Konishi K, Mizutani H, Yamada N, Isaka N, Nakano T, Ito M. High glucose induces plasminogen activator inhibitor-1 expression through Rho/Rho-kinase-mediated NF-kappaB activation in bovine aortic endothelial cells. Atherosclerosis. 2008;196:22–28. doi: 10.1016/j.atherosclerosis.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Rippe A, Rippe B. Acute hyperglycemia induces rapid, reversible increases in glomerular permeability in nondiabetic rats. Am J Physiol Renal Physiol. 2010;298:F1306–F1312. doi: 10.1152/ajprenal.00710.2009. [DOI] [PubMed] [Google Scholar]
- Zhou H, Zhang KX, Li YJ, Guo BY, Wang M, Wang M. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses high glucose-induced proliferation and collagen synthesis in rat cardiac fibroblasts. Clin Exp Pharmacol Physiol. 2011;38:387–394. doi: 10.1111/j.1440-1681.2011.05523.x. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Maynard M, Greiner DL, Whittaker P. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal. 2011;14:781–790. doi: 10.1089/ars.2010.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- Shi-Ting W, Mang-Hua X, Wen-Ting C, Feng-Hou G, Zhu-Ying G. Study on tolerance to ischemia-reperfusion injury and protection of ischemic preconditioning of type 1 diabetes rat heart. Biomed Pharmacother. 2011;1:56–60. doi: 10.1016/j.biopha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- Yang MK, Lee SH, Seo HW, Yi KY, Yoo SE, Lee BH, Chung HJ, Won HS, Lee CS, Kwon SH, Choi WS, Shin HS. KR-31761, a novel K + (ATP)-channel opener, exerts cardioprotective effects by opening both mitochondrial K + (ATP) and Sarcolemmal K + (ATP) channels in rat models of ischemia/reperfusion-induced heart injury. J Pharmacol Sci. 2009;109:222–232. doi: 10.1254/jphs.08132FP. [DOI] [PubMed] [Google Scholar]
- Chen ZC, Cheng YZ, Chen LJ, Cheng KC, Li Y, Cheng J. Increase of ATP-sensitive potassium (KATP) channels in the heart of type-1 diabetic rats. Cardiovasc Diabetol. 2012;11:8. doi: 10.1186/1475-2840-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]