Abstract
The multifunctional E3 ubiquitin ligase CHIP is an essential interacting partner of HSP70, which together promote the proteasomal degradation of client proteins. Acute CHIP overexpression provides neuroprotection against neurotoxic mitochondrial stress, glucocorticoids, and accumulation of toxic amyloid fragments, as well as genetic mutations in other E3 ligases, which have been shown to result in familial Parkinson's disease. These studies have created a great deal of interest in understanding CHIP activity, expression and modulation. While CHIP knockout mice have the potential to provide essential insights into the molecular control of cell fate and survival, the animals have been difficult to characterize in vivo due to severe phenotypic and behavioral dysfunction, which have thus far been poorly characterized. Therefore, in the present study we conducted a battery of neurobehavioral and physiological assays of adult CHIP heterozygotic (HET) mutant mice to provide a better understanding of the functional consequence of CHIP deficiency. We found that CHIP HET mice had normal body and brain weight, body temperature, muscle tone and breathing patterns, but do have a significant elevation in baseline heart rate. Meanwhile basic behavioral screens of sensory, motor, emotional and cognitive functions were normative. We observed no alterations in performance in the elevated plus maze, light-dark preference and tail suspension assays, or two simple cognitive tasks: novel object recognition and spontaneous alternation in a Y maze. Significant deficits were found, however, when CHIP HET mice performed wire hang, inverted screen, wire maneuver, and open field tasks. Taken together, our data indicate a clear subset of behaviors that are altered at baseline in CHIP deficient animals, which will further guide whole animal studies of the effects of CHIP dysregulation on cardiac function, brain circuitry and function, and responsiveness to environmental and cellular stress.
Introduction
Heat shock proteins (HSPs) are highly conserved, abundantly expressed chaperone proteins with diverse functions, including the assembly of multi-protein complexes, transport of nascent polypeptides, regulation of protein folding, and neuroprotection [1]. Heat shock protein 70 (HSP70) is the major stress-inducible chaperone in the CNS and is upregulated with thermal stress, oxidative injury, and after various acute and chronic insults [2], [3], [4]. HSP70 operates as part of a multi-protein complex in which associated co-chaperone molecules can alter client proteins bound to HSP70 thereby affecting the trafficking, degradation and refolding activities of HSP70 [5], [6], [7]. The multifunctional E3 ubiquitin ligase, C-terminus of heat shock cognate 70 interacting protein (CHIP) is a key interacting protein of HSP70, and appears to primarily promote client protein ubiquitination and subsequent proteasomal degradation [5], [6].
Over 30 proteins have been identified as specific substrates of CHIP, many of which are associated with cellular differentiation and survival, as well as susceptibility to stress [5]. CHIP is a central modulator of both the activity and expression of HSP70 as it interacts with the chaperone and also promotes its degradation [8]. Other CHIP client proteins have central roles in glucocorticoid response, tau degradation, and both p53 and cAMP signaling [9], [10], [11], [12], [13], [14]. Loss of CHIP might result in increased susceptibility to stress and increased cell death due to a dysregulation of essential client proteins. Indeed, overexpression of CHIP can decrease central nervous system cell death in models of chronic neurodegenerative diseases, including Parkinson's and Alzheimer's disease [11], [15], [16]. Our group has, however, recently shown that CHIP can affect essential signaling pathways and be deleterious to cellular health when chronically overexpressed [17], suggesting a delicate balance must exist to promote cell survival.
In order to better understand the role of CHIP as a mediator of the chaperone response, mice that are deficient in CHIP expression were developed by Patterson and colleagues [18], [19]. Knockout mice weigh 30% less than wild type (WT) mice at 3 and 12 months of age, have increased and accelerated skeletal muscle atrophy, and possess significantly decreased body fat stores at 12 months. The authors also found that CHIP deficient mice have larger hearts at 12 months of age, indicative of cardiac hypertrophy. Together with the fact that CHIP knockout animals exhibit signs of osteoporosis and kyphosis, these data suggest an advanced-ageing phenotype and also help to explain the significantly decreased lifespan of these animals [18], [19].
We hypothesize that cellular and circuit adaptations and maladaptations to CHIP or other chaperone molecules contributes to individual vulnerabilities that may result in the development of neurodegenerative disorders and/or increased risk for neurological dysfunction following ischemic events [20], [21]. While the role of oxidative and nitrosylative stress and CNS degeneration is well appreciated [22], these pathways may also contribute to the more subtle manifestations of diseases like Parkinson's and Alzheimer's which have an affective component including comorbid depression. Indeed, links between cellular stress responses and vulnerability to stress-related mental health disorders has also been recently postulated [23], [24].
To test this hypothesis we examine the effects of CHIP haploinsufficiency on behavioral and neurological function in mice similar to previous studies of other large impact brain mutations, such as those in Reelin [25], [26], Scrapper [27], and monoamine transporters [28]. In this work, we report the presence of specific motor disturbances in CHIP heterozygous (HET) mice in the absence of changes in emotional reactivity, learning and memory, or behavioral despair.
Materials and Methods
Animals and General Behavioral Methods
CHIP (also known as Stub1) heterozygous and wild type littermate mice were bred in house from matings of heterozygous to wild type mice, and weaned at postnatal day (P) 21 at which time genotypes were determined by PCR analysis of tail tissue using standard procedures [18], [19]. Mice were housed 2–5 per cage in a temperature and humidity controlled environment (lights on 0600-1800 h) within AAALAC approved Vanderbilt University Animal Care Facilities. Food and water were available ad libitum. All procedures were approved by the Animal Care and Use Committee at Vanderbilt University. Mice were extensively handled for at least one week prior to the beginning of experiments, were at least P70 prior to testing, and were habituated to the testing rooms for ∼30 min prior to testing. Only male mice were tested. Testing apparatuses were cleaned with Vimoba spray (Quip Labs, Wilmington, DE) between animals and wiped with paper towels to clear the solution and any debris. Mice were weighed prior to and at the conclusion of behavioral testing (no significant changes). After behavioral testing, mice were euthanized with an overdose of isoflurane and brains were removed and weighed.
Wire Hang
Gross motor function was examined in a basic wire hang test. Mice were allowed to hang via their forepaws on a plastic wire (14 gauge) suspended 35 cm above the surface. Mice were able to use their back paws for balance and support during the test. Latency to fall into an arena with fresh bedding was recorded for up to 180 sec, at which point mice were removed from the wire and returned to the home cage.
Inverted Screen
For the inverted screen test, 2–4 littermates were placed on a metal grid screen (10 cm×14 cm) with separate compartments. After placement, the grid was inverted 60 cm over a plastic cage containing fresh bedding. Latency to fall was recorded for up to 60 sec, at which point mice were removed from the apparatus and returned to the home cage. Two trials were conducted on separate days, and data averaged together.
Grip Strength
Grip force was measured using a force gauge (San Diego Instruments, San Diego, CA) [29]. Five trials were carried out consecutively. Testing was conducted in normal lighting conditions (250–300 lux). Grip strength was recorded via a digital gauge that locked in the peak reading of each trial. The average grip strength score, given in Newtons, is the average of five trials.
Rotarod
Motor coordination and balance were measured using a commercially available accelerating rotarod apparatus (Ugo Basile model 7650) as previously described [30]. Mice were placed on the rotating cylinder (3 cm in diameter) and confined to a section approximately 6.0 cm long by gray plastic dividers. The rotational speed of the cylinder was increased from 5 to 40 r.p.m. over a 5 min period. Latency at which mice fell off the rotating cylinder was measured. Each mouse was given three trials per day over a period of 3 days.
Cutaneous Mechanical Sensitivity
Mice were lowered by their tails into a small box with wire mesh flooring, which is raised approximately 45 cm above the table surface. Von Frey filaments (North Coast Medical, Inc., Morgan Hill, CA) were applied perpendicularly against the planar surface of a back paw until bowing of the fiber occurred in ascending order of thickness. The bent filament was held for 2 sec and applied five times at 5 sec intervals. The test ended when the mouse performed three withdrawal responses out of five applications of the filament, with a withdrawal response being qualified as the paw being entirely removed from the mesh flooring in a motion not related to general locomotion.
Elevated Plus Maze (EPM)
Anxiety responses were examined using a custom EPM (White, 67 cm length×6.5 cm width×15 cm height in the closed arms). The entire apparatus was elevated 40 cm from the ground and testing was conducted under normal lighting (250–300 lux). At the start of the 5 min trial, each mouse was lowered, by its tail, onto an open section of the maze, directly next to and facing a closed section. Sessions were recorded by a ceiling-mounted video camera connected to a computer using video acquisition and analysis software (ANY-maze, Stoelting, Wood Dale, IL). Data analyzed included % open arm time, number of zone crossings and distance traveled throughout the maze [31], [32].
Y maze
Spontaneous alternation was examined using a 3-arm Y maze (6×35.5×10 cm) under normal lighting (250–300 lux). At the start of the 6 min trial, each mouse was lowered, by its tail, into one arm of the maze, facing towards the center junction. Sessions were recorded by a ceiling-mounted video camera connected to a computer using video acquisition and ANY-maze analysis software (Stoelting, Wood Dale, IL). Data analyzed included number of arm entries and sequence of entries, allowing calculation of % spontaneous alternation (consecutive entry into each of the three arms), as described previously [32], [33].
Open Field Activity
Locomotor activity in a novel open field was measured using commercial open field activity chambers (Med Associates, 27×27×20.5 cm) that were contained within a light- and air-controlled environment (Med Associates, 64×45×42 cm). Location and movement were detected by the interruption of infrared beams by the body of the mouse (16 photocells in each horizontal direction, as well as 16 photocells elevated 4 cm to measure rearing) and were measured by the Med Associates Activity Monitor program [34]. Activity was measured for a 90 min session.
Light-Dark Preference
The light-dark preference test uses an opaque insert placed within one-half of the Med Associates Open Field chambers. The dark insert measures 27 cm in length, 14 cm in width, and 20.5 cm in height, with a single door for entering/exiting a darkened area and is placed on the left side of the activity chamber. Movement and location are again detected by the interruption of infrared beams during a 10 min session.
Novel Object Recognition
Nonspatial working memory was assessed in a two object recognition task [32], [33]. The day before testing, mice were habituated to the novel object arena (39.5×28.5×19.5 cm, Allentown, Inc., Allentown, NJ) for 20 min. On the test day, mice were placed into the cage with two identical objects (either 50 ml conical tubes weighted with sand or small blue microscope slide boxes on their side) placed on the two sides of the cage for 5 min. After a 10 min delay in their home cage they were returned to the arena and allowed to explore two objects, one identical to the original objects and the other different, for an additional 5 min. Time spent exploring novel versus familiar objects was recorded by post-hoc video analysis. A counter-balanced design was used to control for side of presentation and which of the pair of objects was novel. Exploration of the two identical objects was compared to verify that there was not a measurable object or side bias despite the experimental design.
Tail Suspension Test (TST)
Behavioral despair was measured by suspending mice for 7 min by the tail from a vertical aluminum bar attached to the top of a box-like enclosure (Med Associates, 33×33×32 cm) that is open in the front. Mice are attached to the bar by tape placed ∼1.5 cm from the tip of the tail. Force transducers and automated software (Med Associates) is used to measure immobility. Settings utilized were a lower threshold of 7, upper threshold of 20, gain of 8, and resolution of 220 ms.
Modified Irwin Screen and Miscellaneous Tests
Mice were screened for basic sensorimotor functions and reflexive measures in a modified Irwin battery [35], with minor modifications as previously described [36]. Oxygen saturation, heart rate and breath rate were measured in awake mice with a MouseOx system (Starr Life Sciences, Oakmont, PA) and CollarClip sensor (size small).
Statistical Analysis
Genotype differences were assessed by unpaired Student's t-test or ANOVA as appropriate with significance defined as two-tailed p<0.05. Analyses of wire maneuver performance and von Frey filament required to produce a withdrawal response were analyzed by chi square (χ2).
Results
General Physiological Variables
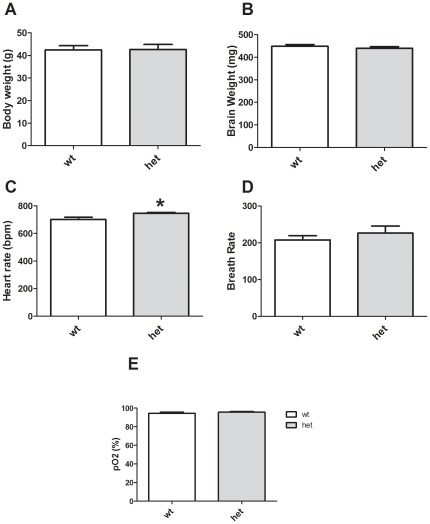
Although CHIP knockout mice exhibit significant weight loss (30% or more), muscle atrophy, bone abnormalities and cardiac hypertrophy [12], [13] (and unpublished observations), HET mice have no obviously clear phenotype upon simple observation. Body and brain weights of CHIP HET mice did not differ from WT littermate controls (Figure 1A, p = 0.944 and 1B p = 0.386). There was a small but significant increase in the heart rate of HET mice (Figure 1C, p = 0.010); however, there were no significant differences in breath rate (Figure 1D, p = 0.459) or blood oxygenation (Figure 1E, p = 0.334) between genotypes. Mice were tested for additional physiological and reflexive measures using a modified Irwin battery, which indicated that the WT and HET genotypes were similar in most measures (Table 1).
Figure 1. Specific physiological alterations in CHIP HET mice.
Body weights (A) and brain weights (B) do not differ between CHIP WT and HET mice. (C) HET mice exhibit increased heart rate, but breath rate (D) and pO2 (E) are not significantly different from WT mice. *p<0.05.
Table 1. Modified Irwin Screen.
| Physical Condition | +/+ | +/− |
| Initial Body Weight (g) | 33.4±1.95 | 34.8±2.07 |
| Final Body Weights (g) | 42.4±1.95 | 42.7±2.25 |
| Final Brain Weight (mg) | 447±7.05 | 442±6.80 |
| Rectal Temperature (°C) | 34.7±0.28 | 35.1±0.29 |
| Normal Presence of Whiskers (%) | 100 | 100 |
| Well groomed (%) | 100 | 100 |
| Piloerection (%) | 0 | 0 |
| Fur missing on face (%) | 0 | 0 |
| Fur missing on body (%) | 0 | 0 |
| Wounds (%) | 0 | 0 |
| Agouti Fur Color (%) | 100 | 100 |
Mice were screened in a battery of measures to assess overall health and basic sensory and reflexive function. Values reflect the mean ± SEM of the percentage of animals exhibiting each attribute unless noted. Sample size: wild type (WT, +/+) n = 11, heterozygous (HET, +/−) n = 12.
p<0.05.
Simple Elicited Sensory and Motor Functions
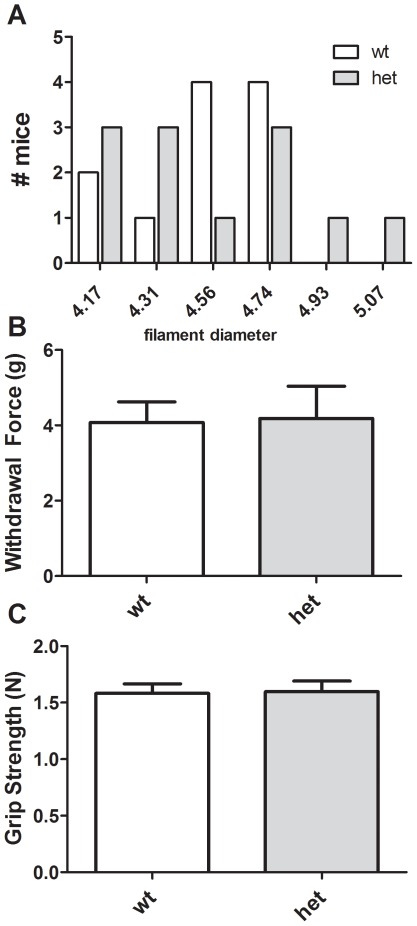
Paw withdrawal responses to somatosensory stimuli were examined using von Frey filaments. HET and WT mice did not differ either when analyzed by distribution of required filament to produce a response (Figure 2A, χ2 (5, N = 23, 5.11, p = 0.403), or by mean withdrawal force required (Figure 2B, p = 0.916). Muscle tone was measured using a grip strength apparatus and again no differences were revealed (Figure 2C, p = 0.908).
Figure 2. Normal somatosensory function and grip strength in CHIP HET mice.
CHIP WT and HET mice exhibit normal paw withdrawal sensitivities to mechanical stimuli using von Frey filaments (A, B) and forepaw grip strength (C).
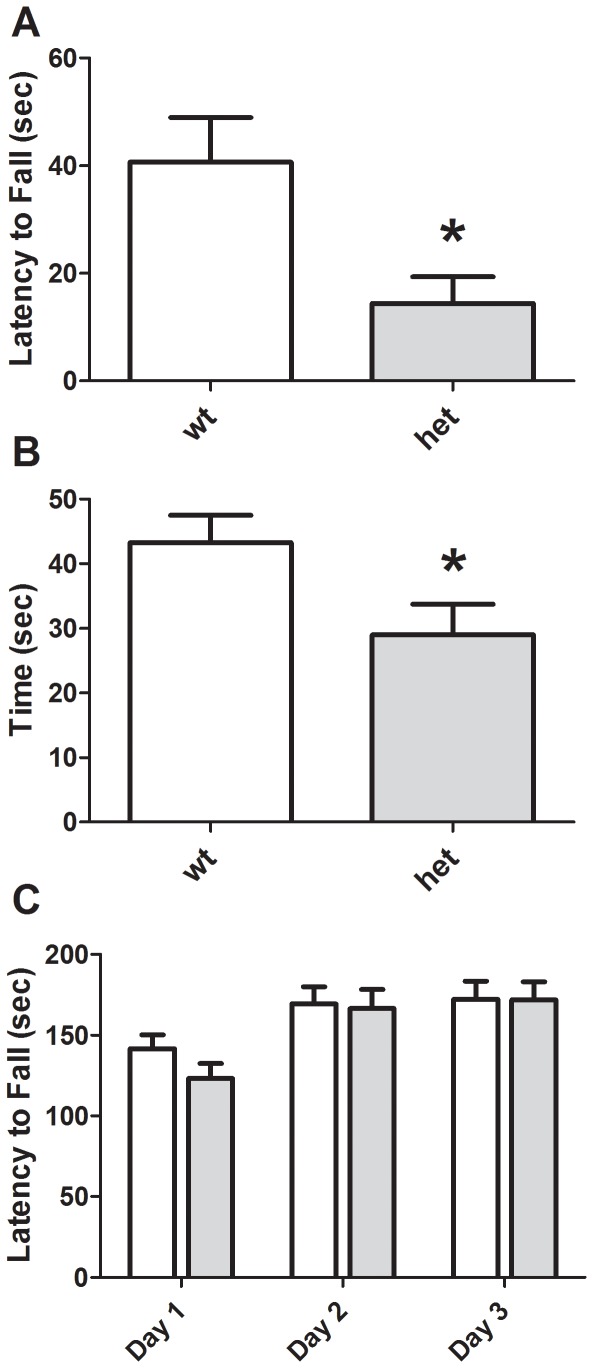
CHIP HET mice exhibited a more rapid latency to fall from a wire hang test (Figure 3A and Table 1, p = 0.012) and inverted screen (Figure 3B and Table 1, p = 0.037), suggesting motor impairments. Success in a wire maneuver task was also impaired in CHIP HET mice (Table 1, χ2 [4, N = 23, 108.8, p<0.0001]). Motor learning, as assessed on an accelerating rotarod, was intact (Figure 3C). Two-way ANOVA revealed a significant effect of trial day, demonstrating the presence of motor learning [F (2, 67) = 3.27, p<0.05]. However, there were no significant differences based on genotype [F (1, 22) = 0.270, p = 0.6050] or genotype×trial day interaction [p = 0.846].
Figure 3. Motor abnormalities in CHIP HET mice.
CHIP HET mice exhibit a reduced latency to fall from a wire hang apparatus (A) and reduced time holding onto an inverted screen (B), but normal performance and learning on an accelerating rotarod (C). *p<0.05.
Motivated and Emotional Behaviors
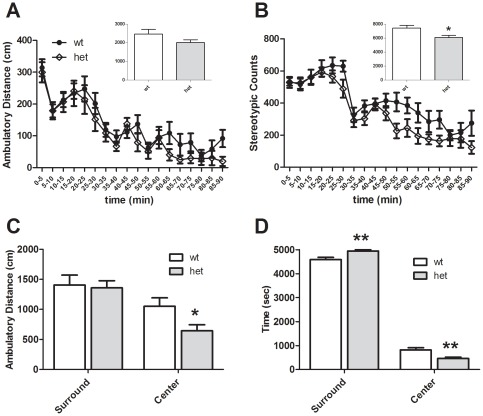
Mice were exposed to a novel open field and locomotor exploration was measured over a 90 min period. HET and WT mice did not significantly differ in ambulatory distance (Figure 4A, p = 0.135) or time (data not shown), but CHIP HET mice did exhibit a small reduction in motor stereotypies (Figure 4B, p = 0.012). Hypoactivity was particularly evident during the later portions of the test (∼60–90 min), suggesting that HET mice might have an increased rate of habituation to the testing environment. Analysis of thigmotaxis revealed that HET mice spent more time in the periphery rather than the center (Figure 4C, p<0.01), suggesting possible increases in anxiety in HET mice.
Figure 4. Open Field behaviors in CHIP WT and HET mice.
CHIP HET mice exhibit a significant decrease in motor stereotypies within an open field apparatus (B), but only a non-significant trend in total ambulatory distance (panel A, p = .135). However, upon separation of the open field into center and surround zones, there is a significant decrease in distance (C) and time spent in the center (D), suggesting an increase in anxiety-related behavior. *p<0.05, **p<0.01.
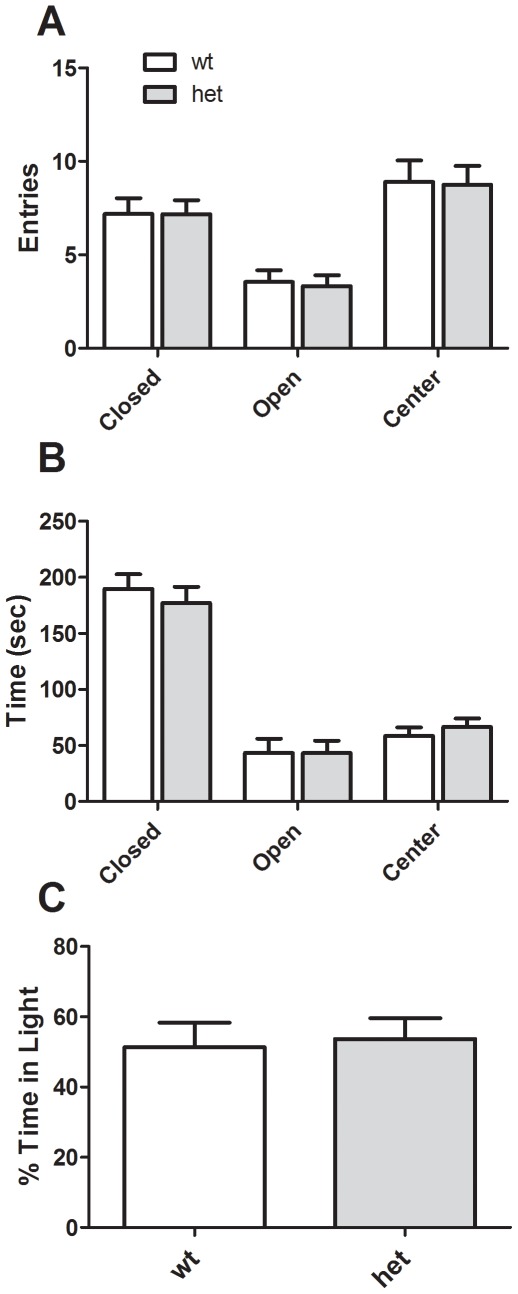
We attempted to confirm an anxiogenic phenotype in CHIP HET mice using additional assays. In an elevated plus maze there was no significant effect of genotype on open arm entries [F (1, 22) = 0.035, p = 0.853] or time [F (1, 22) = 0.025, p = 0.874] (Figures 5A and B, respectively). As expected, there were significant effects of arm location, with more entries to the center and closed arms [F (2, 67) = 21.3, p<0.001] and a strong time preference for closed arms [F (2, 67) = 87.7, p<0.001]. We also measured protected and unprotected head dips and found no effect of genotype (data not shown). Finally, light-dark preference was unchanged (p = 0.803; Figure 5C), as was the latency to enter the dark zone for the first time (data not shown, p = 0.742).
Figure 5. Anxiety-related behaviors in CHIP HET mice.
Neither the number of arm entries (A) nor time spent in arms (B) in an elevated plus maze demonstrates differences in anxiety responses. Similarly, there are no differences between CHIP WT and HET mice in a light-dark preference assay (C).
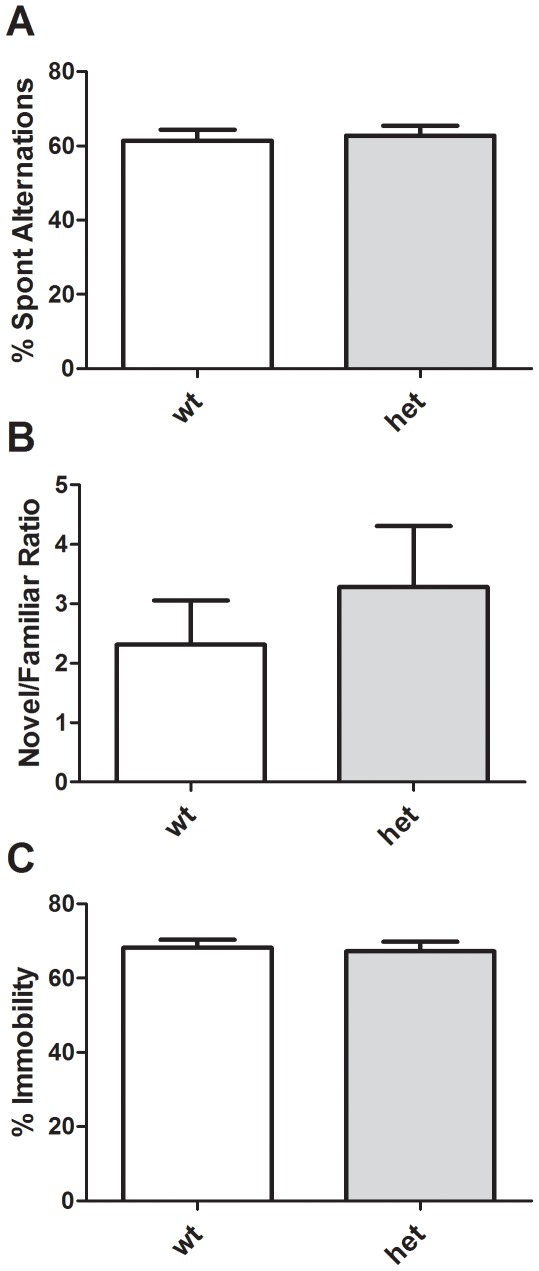
Spatial working memory was studied using spontaneous alternation in a Y maze. There were no significant differences in total arm entries (data not shown), or percent alternation (Figure 6A, p = 0.744). Nonspatial memory was assessed using a novel-object recognition paradigm and again no differences between genotypes were noted (Figure 6B, p = 0.450). Finally, mice were assessed for behavioral despair using the tail suspension test (Figure 6C), and no significant differences in immobility between genotypes were noted (p = 0.787).
Figure 6. Cognitive and depressive behaviors in CHIP HET mice.
No differences between CHIP WT and HET mice were noted in Y maze spontaneous alternation (A), novel object recognition (B) or tail suspension test (C) assays.
Taken together, these studies indicate modest impairments in motor function, cardiovascular function and possibly anxiety status in CHIP haploinsufficient mice.
Discussion
The E3 ubiquitin ligase CHIP binds to HSC70 and HSP70 and critically controls expression of both HSP70 and other essential client proteins, a feature which is more prominent when CHIP expression increases as a consequence of acute injury [15], [17], [37], [38], [39]. CHIP knockout mice are increasingly being used to examine neuroprotection and the molecular control of cell fate [17], [18], [19]. However, CHIP null mice die prematurely, with significant mortality observed in the peripartum and early postnatal periods, and exhibit a host of severe neurological and peripheral phenotypes [19], including profound ataxia and growth impairments. In contrast, a single CHIP allele disruption results in a 50% loss of CHIP expression, but no overt phenotype [19]. Use of heterozygotic mutants allows for clearer interpretation of abnormal phenotypes, and likely a better translation to human disease given that, at least at this time, no total loss-of-function mutations in CHIP have been observed in human disease states. In the present study, therefore, we conducted a battery of neurobehavioral and physiological experiments to provide a better understanding of the functional consequences of CHIP haploinsufficiency.
CHIP HET mice exhibited normal survival, growth rates, body temperature, grooming, muscle tone and breathing patterns. There was a small, but statistically significant elevation of heart rate in the mutant mice. As heart rate was measured in awake mice, it is possible that the increase in heart rate is a physiological manifestation of behavioral stress. Alternatively, this may indicate an intermediate phenotype to the significant cardiac hypertrophy observed in CHIP knockout mice. Somatosensory responses, grip strength, pole climbing and coordination (i.e. rotarod performance) were also normal, but some aspects of motor control were deficient in the CHIP HET animals. The haploinsufficient mice fell more quickly from both a wire and an inverted screen and had difficulty using their hind paws in a wire maneuver task.
When placed in a novel open field, CHIP HET mice exhibited significantly less motor stereotypies than control animals and traversed less horizontal distance specifically in the center of the arena. Thus, the CHIP mutants spent more time near the edges of the chamber, rather than in the more open center portion. This phenotype is generally thought to reflect an increase in anxiety state, although there are other possible explanations [40]. We also noted that the CHIP HET mice were more timid during routine handling procedures reflected by an increased startle response (unpublished observations) [41], which was surprising given that when animals were examined in the elevated plus maze and light-dark tasks, no alterations in anxiety behaviors were apparent. Expression of behavioral despair in a tail suspension assay was also normal. Taken together, these observations suggest that CHIP HET mice may express subtle context specific alterations in anxiety and/or stress reactivity that were not captured by our testing. Future studies using additional testing paradigms and neuroendocrine measures will be necessary to explore this further.
We employed spontaneous alternation in a Y maze as a basic screen for spatial working memory [32], [33], but observed no significant changes. Rates of spontaneous alternation are also influenced by attention, anxiety and arousal state (for reviews, see [42], [43]). Similarly, a novel object recognition paradigm was used to explore short-term nonspatial working memory [32], [33], but no alterations were noted. Long-term learning and/or memory may be affected in the mutant mice, but has not been examined at this time. It is also worth noting that all of our studies were conducted in young adult mice; it is possible that CHIP mutant mice exhibit premature age-related declines in performance in these tasks.
Mice heterozygotic for another E3 ubiquitin ligase Scrapper, have also recently been behaviorally characterized [27] and it is worth noting some parallels between these mice and CHIP haploinsufficient mice. Scrapper is one of a group of E3 ligases with essential roles in neurite outgrowth. The protein is localized to synapses and Scrapper null mice die at birth. The heterozygous mice are viable and grossly normal. Detailed analyses suggest normal motor function and anxiety level through elevated plus maze and light-dark tests; however, Scrapper HET mice show changes in center-surround activity patterns in an open field, with increased center time [27]. In the current study, we again see context-specific effects on anxiety measures, but CHIP HET mice exhibit increased anxiety in an open field. Similar to the CHIP mutants, Scrapper HET mice also showed normal sensory responses and were not different from controls in most learning and memory paradigms. Scrapper HET mice, however, did exhibit impaired contextual fear conditioning (but not cue) [27], suggesting deficits in some aspects of hippocampal function. It will be very interesting to assess fear conditioning in CHIP HET mice in the future. Social approach and aggression would also be interesting domains to explore [44], [45], [46].
It has been hypothesized that protein dysregulation and increased accumulation of dysfunctional organelles, as well as proteins, result in late-onset neurodegeneration [15], [47]. Mice with a full deletion of the E3 ligase Parkin have remarkably subtle pathological and behavioral changes [48], [49], although mutations in the PARK2 gene, coding for Parkin, constitute the most common familial form of PD [50]. CHIP overexpression compensates for loss of Parkin activity suggesting that these proteins have homologous functions and client proteins. Our data taken with that of our colleague Dr. Patterson, who initially characterized this mutant mouse, suggest that CHIP plays a more central role in CNS and cardiac cell stress and survival. Given that CHIP function and expression in the brain decline during aging suggests that decreased levels of CHIP are sufficient to induce dysfunctions that might be associated to human neurodegenerative diseases. Once we move CHIP deficiency from the hybrid B6/C57 to their 10th generation of breeding on the B6 background, we will confirm these behavioral traits and pursue analysis of CNS pathology in homozygote and heterozygote animals [51], [52].
In summary, our data point to the presence of specific motor disturbances in CHIP HET mice, suggesting that moderate underexpression of CHIP produces functional impairments in brain circuits. In contrast, we observed only a very modest anxiety phenotype and no changes in performance in simple tasks of behavioral despair and learning and memory. Taken together, these data indicate a relatively subtle but clear phenotype of CHIP haploinsufficiency which will help guide future studies of the effects of CHIP dysregulation on brain circuitry, function, and neuronal responsiveness to environmental and cellular stress.
Acknowledgments
The authors wish to thank Heather Durai and A. Cozette Kale for assistance with animal generation and genotyping and Dr. John Allison for support services within the Neurobehavioral Core. Dr. Cam Patterson (UNC, Chapel Hill) generously donated CHIP mutant mice from which our colony was generated.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Financial support was provided by a grant from the JB Marshall Neurovascular Therapeutics Program at Vanderbilt University (B.M.), the Vanderbilt Clinical Neuroscience Scholars Program (A.M.P.), the Vanderbilt Center for Molecular Neuroscience, the Vanderbilt Kennedy Center and the Vanderbilt Brain Institute (A.M.P.). Behavioral work was performed at the Vanderbilt Mouse Neurobehavioral Core, which is supported in part by P30HD15052. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1.Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Duffy AE, Bordelon YM, McLaughlin B. Killer proteases and little strokes–how the things that do not kill you make you stronger. J Cereb Blood Flow Metab. 2007;27:655–668. doi: 10.1038/sj.jcbfm.9600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 5.Arndt V, Rogon C, Höhfeld J. To be, or not to be — molecular chaperones in protein degradation. Cellular and Molecular Life Sciences. 2007;64:2525–2541. doi: 10.1007/s00018-007-7188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohfeld J, Cyr DM, Patterson C. From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep. 2001;2:885–890. doi: 10.1093/embo-reports/kve206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian SB, McDonough H, Boellmann F, Cyr DM, Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440:551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundrat L, Regan L. Identification of residues on Hsp70 and Hsp90 ubiquitinated by the cochaperone CHIP. Journal of Molecular Biology. 2010;395:587–594. doi: 10.1016/j.jmb.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonough H, Charles PC, Hilliard EG, Qian S-B, Min J-n, et al. Stress-dependent CHIP/DAXX interaction suppresses the p53 apoptotic program. Journal of Biological Chemistry. 2009:20649–20659. doi: 10.1074/jbc.M109.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. Journal of Biological Chemistry. 2004;279:4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 11.Sahara N, Murayama M, Mizoroki T, Urushitani M, Imai Y, et al. In vivo evidence of CHIP up-regulation attenuating tau aggregation. Journal of Neurochemistry. 2005;94:1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 12.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Human Molecular Genetics. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q-g, Han D, Wang R-m, Dong Y, Yang F, et al. C terminus of Hsc70-interacting protein (CHIP)-mediated degradation of hippocampal estrogen receptor-α and the critical period hypothesis of estrogen neuroprotection. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1104391108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan M, Park A, Nephew KP. CHIP (Carboxyl Terminus of Hsc70-Interacting Protein) promotes basal and geldanamycin-induced degradation of estrogen receptor- alpha. Molecular Endocrinology (Baltimore, Md) 2005;19:2901. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- 15.Dickey CA, Patterson C, Dickson D, Petrucelli L. Brain CHIP: removing the culprits in neurodegenerative disease. Trends in Molecular Medicine. 2007;13:32. doi: 10.1016/j.molmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, et al. CHIP Is Associated with Parkin, a gene responsible for familial Parkinson's Disease, and enhances its ubiquitin ligase activity. Molecular Cell. 2002;10:55–67. doi: 10.1016/s1097-2765(02)00583-x. [DOI] [PubMed] [Google Scholar]
- 17.Stankowski JN, Zeiger SL, Cohen EL, DeFranco DB, Cai J, et al. C-terminus of heat shock cognate 70 interacting protein increases following stroke and impairs survival against acute oxidative stress. Antioxid Redox Signal. 2011;14:1787–1801. doi: 10.1089/ars.2010.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Q, Zhang C, Wu Y, McDonough H, Whaley RA, et al. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003;22:5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min J-N, Whaley RA, Sharpless NE, Lockyer P, Portbury AL, et al. CHIP Deficiency Decreases Longevity, with Accelerated Aging Phenotypes Accompanied by Altered Protein Quality Control. Mol Cell Biol. 2008;28:4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeiger SLH, Musiek ES, Zanoni G, Vidari G, Morrow JD, et al. Neurotoxic lipid peroxidation species formed by ischemic stroke increase injury. Free Radical Biology and Medicine. 2009;47:1422–1431. doi: 10.1016/j.freeradbiomed.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaughlin B, Levitt P. Molecular basis of neurological disease.; In: Wong-Reiley MTT, editor. Philadelphia: Hanley and Belfus Inc; 2000. [Google Scholar]
- 22.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 23.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 24.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res. 2010;68:261–275. doi: 10.1016/j.neures.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, et al. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Laviola G, Ognibene E, Romano E, Adriani W, Keller F. Gene-environment interaction during early development in the heterozygous reeler mouse: clues for modelling of major neurobehavioral syndromes. Neurosci Biobehav Rev. 2009;33:560–572. doi: 10.1016/j.neubiorev.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Yao I, Takao K, Miyakawa T, Ito S, Setou M. Synaptic E3 ligase SCRAPPER in contextual fear conditioning: extensive behavioral phenotyping of Scrapper heterozygote and overexpressing mutant mice. PLoS One. 2011;6:e17317. doi: 10.1371/journal.pone.0017317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalueff AV, Ren-Patterson RF, Murphy DL. The developing use of heterozygous mutant mouse models in brain monoamine transporter research. Trends Pharmacol Sci. 2007;28:122–127. doi: 10.1016/j.tips.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, et al. Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis. 2010;39:169–180. doi: 10.1016/j.nbd.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, et al. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6:411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 31.Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, et al. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustin RM, Shonesy BC, Robinson SL, Rentz TJ, Baucum AJ, 2nd, et al. Loss of Thr286 phosphorylation disrupts synaptic CaMKIIalpha targeting, NMDAR activity and behavior in pre-adolescent mice. Mol Cell Neurosci. 2011;47:286–292. doi: 10.1016/j.mcn.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson BL, Levitt P, Stanwood GD. Prenatal cocaine exposure specifically alters spontaneous alternation behavior. Behav Brain Res. 2005;164:107–116. doi: 10.1016/j.bbr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Stanwood GD, Levitt P. Waved-1 mutant mice are hypersensitive to the locomotor actions of cocaine. Synapse. 2007;61:259–262. doi: 10.1002/syn.20364. [DOI] [PubMed] [Google Scholar]
- 35.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 36.Olsen CM, Childs DS, Stanwood GD, Winder DG. Operant sensation seeking requires metabotropic glutamate receptor 5 (mGluR5). PLoS One. 2010;5:e15085. doi: 10.1371/journal.pone.0015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, et al. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonough H, Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–308. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 41.Whitney G. Timidity and fearfulness of laboratory mice: An illustration of problems in animal temperament. Behavior Genetics. 1970;1:77–85. doi: 10.1007/BF01067373. [DOI] [PubMed] [Google Scholar]
- 42.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 44.Couppis MH, Kennedy CH, Stanwood GD. Differences in aggressive behavior and in the mesocorticolimbic DA system between A/J and BALB/cJ mice. Synapse. 2008;62:715–724. doi: 10.1002/syn.20545. [DOI] [PubMed] [Google Scholar]
- 45.Carter MD, Shah CR, Muller CL, Crawley JN, Carneiro AM, et al. Absence of preference for social novelty and increased grooming in integrin beta3 knockout mice: initial studies and future directions. Autism Res. 2011;4:57–67. doi: 10.1002/aur.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friguet B. Oxidized protein degradation and repair in ageing and oxidative stress. FEBS Lett. 2006;580:2910–2916. doi: 10.1016/j.febslet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 48.von Coelln R, Thomas B, Savitt JM, Lim KL, Sasaki M, et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proceedings of the National Academy of Sciences. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbas N, Lucking CB, Ricard S, Durr A, Bonifati V, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. Human Molecular Genetics. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- 51.Tsvetkov P, Adamovich Y, Elliott E, Shaul Y. E3 ligase STUB1/CHIP regulates NAD(P)H:quinone oxidoreductase 1 (NQO1) accumulation in aged brain, a process impaired in certain Alzheimer disease patients. J Biol Chem. 2011;286:8839–8845. doi: 10.1074/jbc.M110.193276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang GR, Cheng XR, Zhou WX, Zhang YX. Age-related expression of STUB1 in senescence-accelerated mice and its response to anti-Alzheimer's disease traditional Chinese medicine. Neurosci Lett. 2008;438:371–375. doi: 10.1016/j.neulet.2008.04.075. [DOI] [PubMed] [Google Scholar]