Fig. 1.
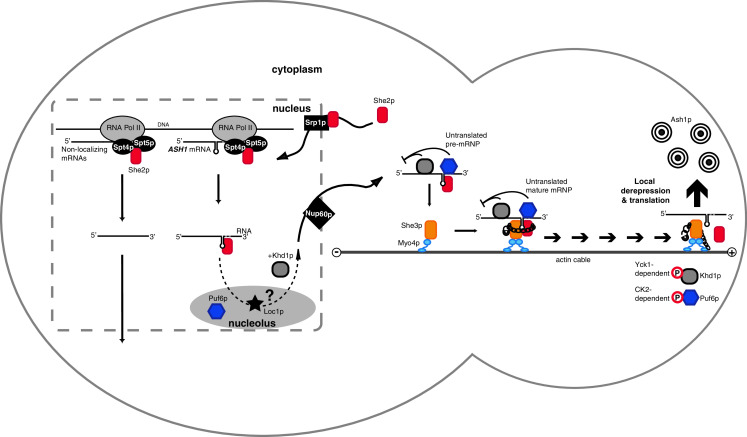
Model of ASH1 mRNA localization in S. cerevisiae. She2p is imported into the nucleus by the importin-alpha Srp1p. At sites of transcription, She2p binds to the elongating RNA polymerase II machinery and associates with nascent mRNAs. Before nuclear export, the ASH1 mRNA-She2p complex transits through the nucleolus, where Loc1p and Puf6p are present. The translational repressors Puf6p and Khd1p are thought to be loaded onto ASH1 mRNA in the nucleolus and in the nucleus, respectively. Efficient nuclear export of localizing mRNAs depends on the specific activity of the nucleoporin Nup60p. Once in the cytoplasm, the ASH1 mRNA-She2p cocomplex binds to Myo4p-associated She3p. Synergistic RNA binding by She2p and She3p dramatically increases the preference for zip-code containing RNAs and ensures that only localizing transcripts are transported. This important quality control step is illustrated by a chain with a padlock. Complex assembly also induces oligomerization of the monomeric Myo4p, which is required for continuous transport of the mRNP. Once the complex is localized at the bud tip, its cargo is translationally activated by phosphorylation of Khd1p and Puf6p. The result is Ash1p expression and inhibition of mating-type switching exclusively in the daughter cell. The mother cell is depicted on the left and the daughter cell on the right. Except for Myo4p, oligomeric states are not depicted. See Fig. 2 for details of ER inheritance
