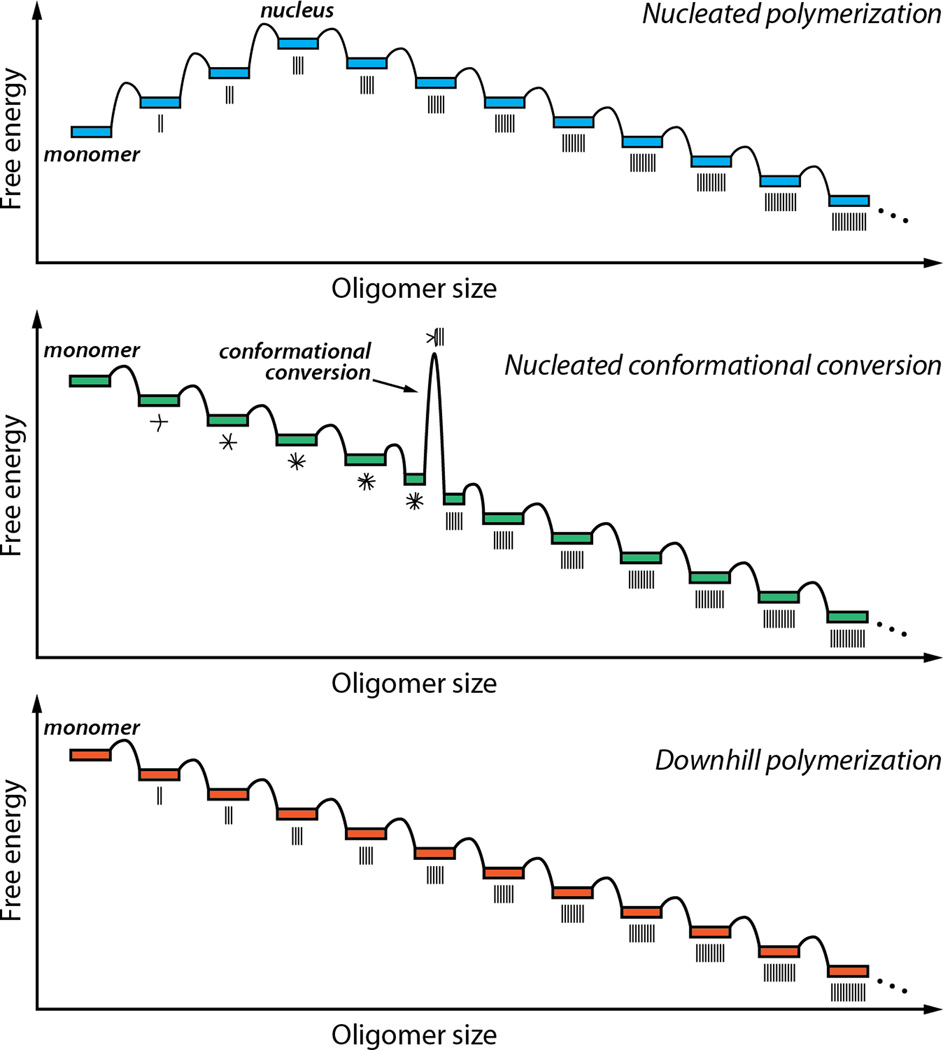
Figure 3.
Energy diagrams associated with three distinct mechanisms of protein aggregation. In a nucleated polymerization (top), the initial association events are unfavorable until a critical sizeis reached. The oligomer of this size is referred to as the nucleus. Subsequent steps are favorable, making further growth favorable for oligomers larger than the nucleus. In a nucleated conformational conversion (middle), facile initial association steps form amorphous oligomers. Oligomers of a certain size can undergo a rate-limiting conversion step, in which they change from an amorphous structure to a cross-β-sheet fibrillar state. Subsequent steps are favorable, as in the nucleated polymerization. In a downhill polymerization (bottom), the mechanism by which TTR aggregates, all of the association steps are favorable after formation of the amyloidogenic intermediate, and there is no kinetic barrier to oligomerization. The aggregates shown are ordered, but they need not be; TTR forms a collection of aggregate structures.