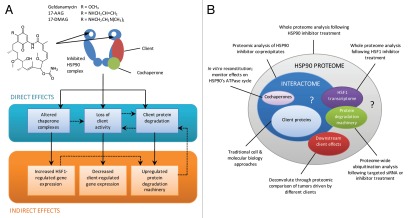
Figure 1.
(A and B) The proteomic response to pharmacological inhibition of HSP90. The molecular chaperone HSP90 functions in the cell as a dimer and interacts with regulatory cochaperones and client proteins. Most pharmacologic HSP90 inhibitors, including all of those currently in clinical trials, bind to the N-terminal nucleotide binding site, thereby inhibiting the essential ATPase activity, interrupting the chaperone cycle and blocking the function of the chaperone. The consequences may be separated into direct effects, e.g., those caused by disruption of the active HSP90 complex, as defined by the interactome, and indirect effects, e.g., downstream alterations to transcriptional programs. Note that inhibitor-induced alteration of HSP90 complexes, such as disruption of client and HSF1 interactions, are believed to lead to many of the indirect transcriptional effects. There is evidence for the interdependence of many aspects of the HSP90 proteome, as indicated by dotted arrows in (A) and overlapping circles in (B).