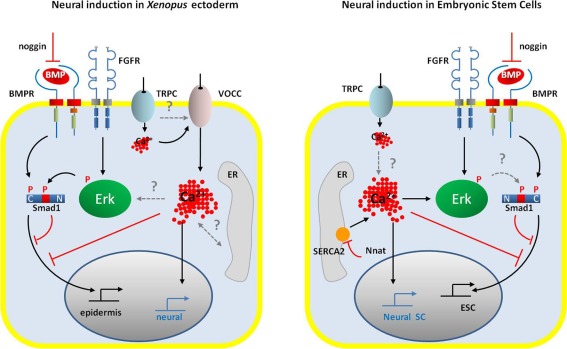
Figure 2.
Schematic representation of the signaling pathways occurring during neural induction in the amphibian ectoderm cells (left panel) and in the ESCs (right panel). In both systems, neural commitment of naïve cells requires the activation of FGF/ERK signaling and the inhibition of BMP signaling by noggin and via the Erk-dependent phosphorylation of Smad1 at linker domain. An increase in intracellular Ca2+ concentration is also a common signal that drives embryonic cells toward the neural fate. However, the control of Ca2+ homeostasis differs between amphibian ectodermal cells and ESCs. While in amphibian, the main source of Ca2+ increase appears to rely on an influx through VOCCs (likely DHP-Ca2+ channels), ESCs do not express VOCC. In ESCs, the regulation of intracellular Ca2+ level depends on the activity of the SERCA2 pump, negatively regulated by neuronatin. Both cell types expressed TRP channels, probably TRPC, which could contribute to the Ca2+ signals. Gating of the VOCC in ectodermal cells could be due to membrane depolarization induced by the activation of TRPC. In ESCs, a direct link between intracellular Ca2+ increase and Erk phosphorylation has been established; in ectodermal cells the question of the amplification of the initial Ca2+ influx by the release of Ca2+ from the ER remains open. However, in both models Ca2+ signals participate in the inhibition of the BMP signaling pathway, either directly or indirectly via Erk-dependent phosphorylation of Smad1. Abbreviations: BMP, bone morphogenetic protein; BMPR, BMP receptor; ER, endoplasmic reticulum; ESC, embryonic stem cell; FGFR, fibroblast growth factor receptor; Nnat, neuronatin; Neural SC, neural stem cell; SERCA2, sarco/endoplasmic reticulum Ca2+-ATPase, isoform2; TRPC, class C transient potential receptor; VOCC, voltage-operated Ca2+ channels.