Abstract
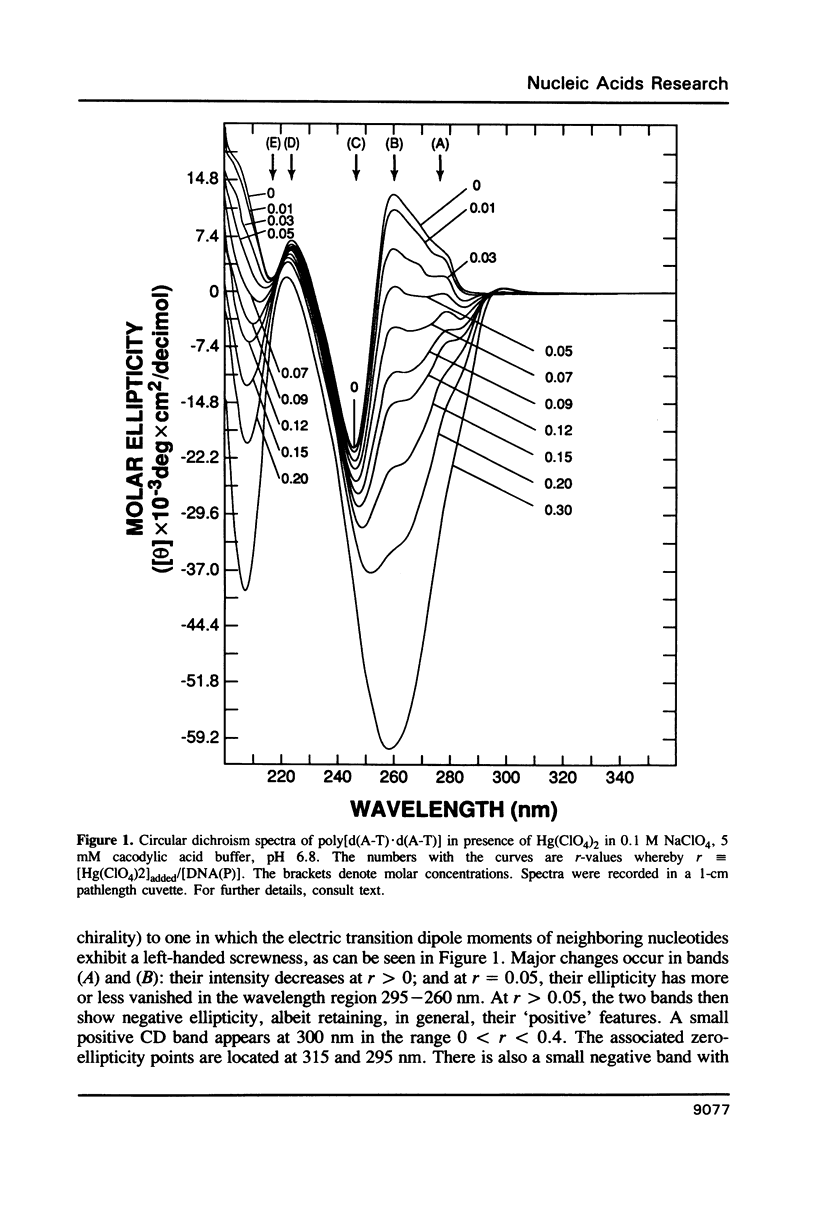
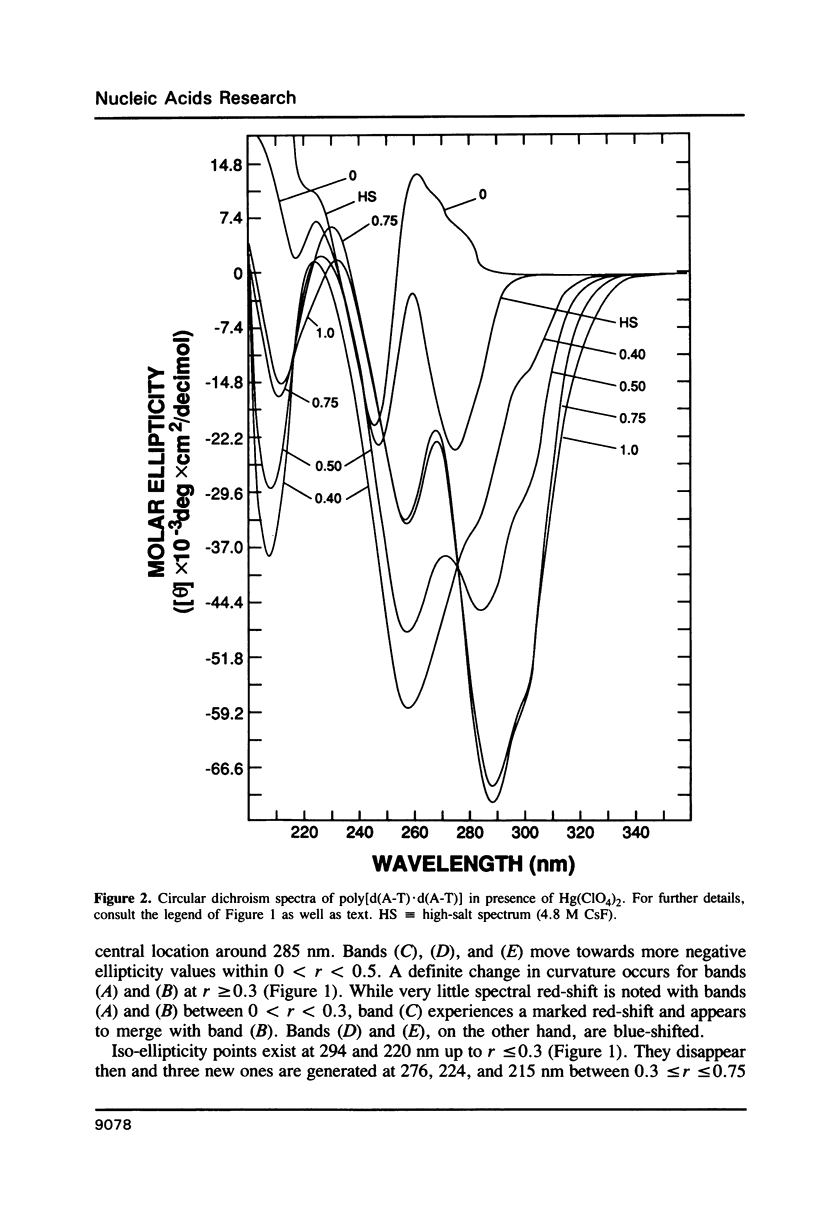
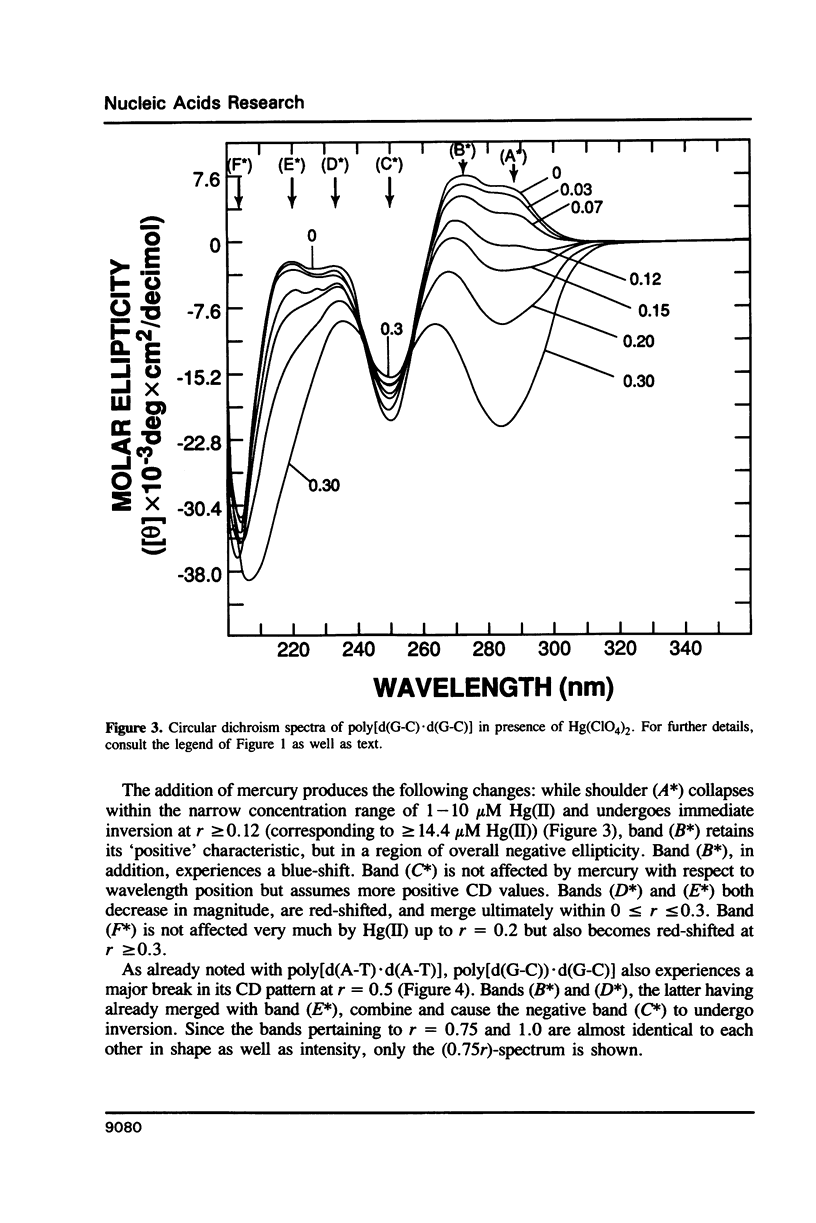
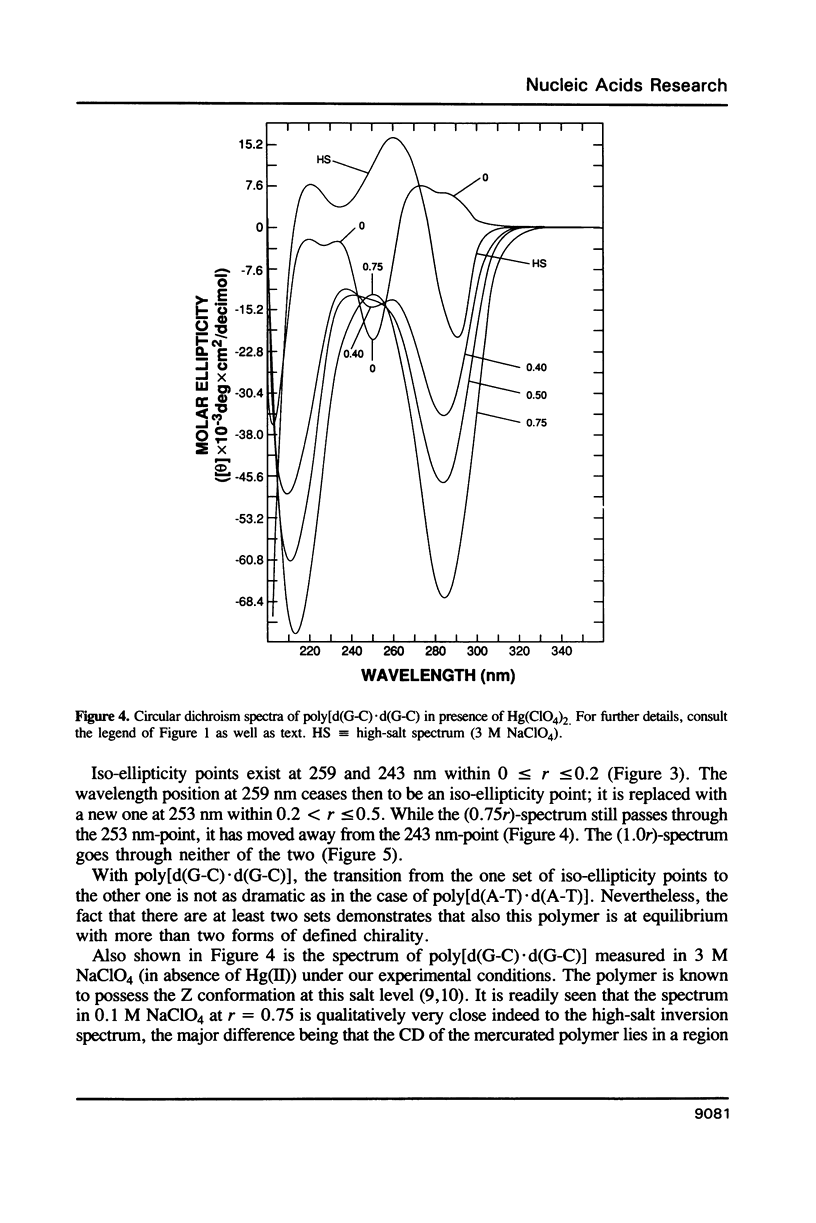
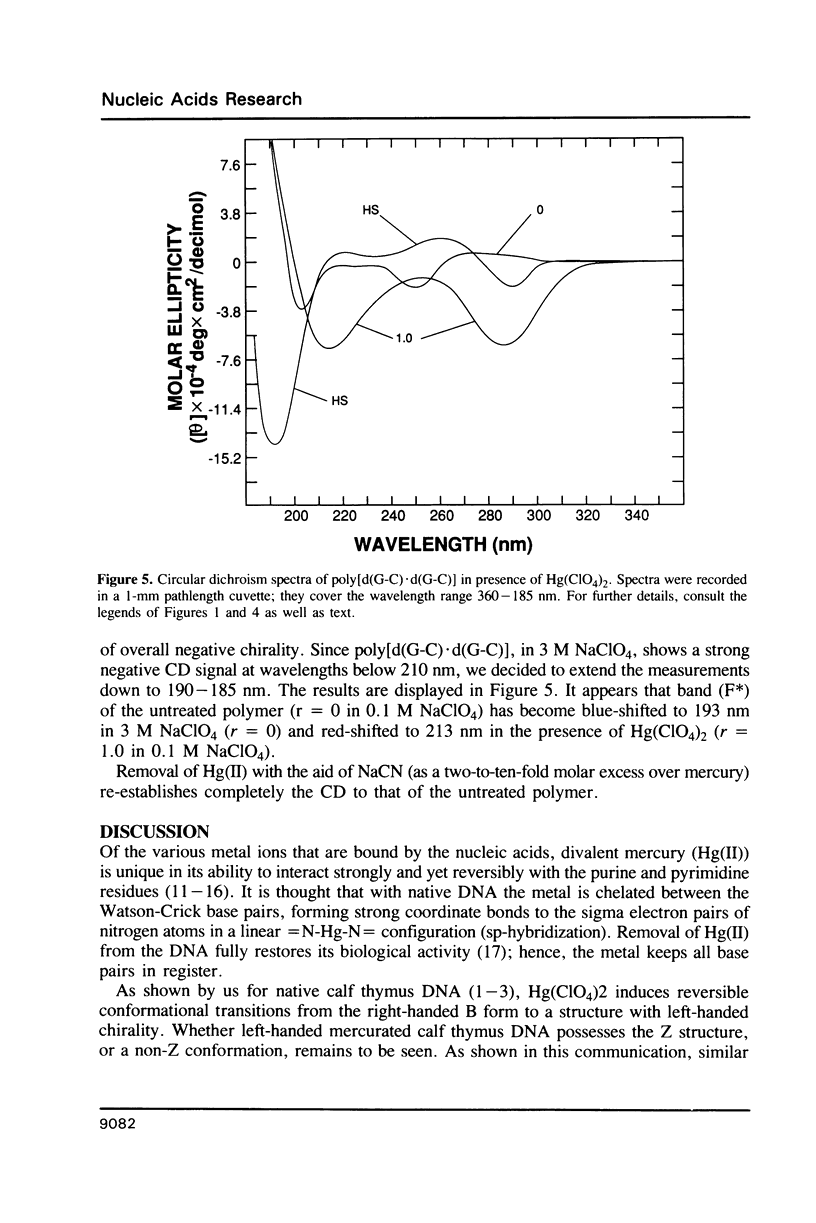
Poly[d(A-T).d(A-T)] and poly[d(G-C).d(G-C)], each dissolved in 0.1 M NaClO4, 5 mM cacodylic acid buffer, pH 6.8, experience inversion of their circular dichroism (CD) spectrum subsequent to the addition of Hg(ClO4)2. Let r identical to [Hg(ClO4)2]added/[DNA-P]. The spectrum of the right-handed form of poly[d(A-T).d(A-T)] turns into that of a seemingly left-handed structure at r greater than or equal to 0.05 while a similar transition is noted with poly[d(G-C).(G-C)] at r greater than or equal to 0.12. The spectral changes are highly cooperative in the long-wavelength region above 250 nm. At r = 1.0, the spectra of the two polymers are more or less mirror images of their CD at r = 0. While most CD bands experience red-shifts upon the addition of Hg(ClO4)2, there are some that are blue-shifted. The CD changes are totally reversible when Hg(II) is removed from the nucleic acids by the addition of a strong complexing agent such as NaCN. This demonstrates that mercury keeps all base pairs in register.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S., Liquier J., Taboury J. A., Taillandier E. Right- and left-handed helixes of poly[d(A-T)].poly[d(A-T)] investigated by infrared spectroscopy. Biochemistry. 1986 Jun 3;25(11):3220–3225. doi: 10.1021/bi00359a021. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg M. S., Gruenwedel D. W. Circular dichroism and ultraviolet absorbance of calf thymus DNA in presence of CH3HgOH. Z Naturforsch C. 1979 Mar-Apr;34(3-4):259–265. [PubMed] [Google Scholar]
- DOVE W. F., YAMANE T. The complete retention of transforming activity after reversal of the interaction of DNA with mercuric ion. Biochem Biophys Res Commun. 1960 Dec;3:608–612. doi: 10.1016/0006-291x(60)90071-1. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Gruenwedel D. W. Circular dichroism of micrococcal nuclease-treated calf thymus chromatin (soluble chromatin) in presence of CH3HgOH. J Inorg Biochem. 1985 Oct;25(2):109–120. doi: 10.1016/0162-0134(85)80019-2. [DOI] [PubMed] [Google Scholar]
- Gruenwedel D. W., Davidson N. Complexing and denaturation of DNA by methylmercuric hydroxide. I. Spectrophotometric studies. J Mol Biol. 1966 Oct 28;21(1):129–144. doi: 10.1016/0022-2836(66)90084-2. [DOI] [PubMed] [Google Scholar]
- Keller P. B., Hartman K. A. Structural forms and transitions for the complex of mercury(II) with poly(dG-dC). J Biomol Struct Dyn. 1987 Jun;4(6):1013–1026. doi: 10.1080/07391102.1987.10507694. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Klysik J., Singleton C. K., Zarling D. A., Jovin T. M., Hanau L. H., Erlanger B. F., Wells R. D. Intervening sequences in human fetal globin genes adopt left-handed Z helices. J Biol Chem. 1984 Jun 10;259(11):7268–7274. [PubMed] [Google Scholar]
- Kollman P., Weiner P., Quigley G., Wang A. Molecular-mechanical studies of Z-DNA: a comparison of the structural and energetic properties of Z- and B-DNA. Biopolymers. 1982 Oct;21(10):1945–1969. doi: 10.1002/bip.360211003. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Riazance J. H., Johnson W. C., Jr, McIntosh L. P., Jovin T. M. Vacuum UV circular dichroism is diagnostic for the left-handed Z form of poly [d(A-C).d(G-T)] and other polydeoxynucleotides. Nucleic Acids Res. 1987 Sep 25;15(18):7627–7636. doi: 10.1093/nar/15.18.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Quasielastic laser light scattering and electron microscopy studies of the conformational transitions and condensation of poly(dA-dT).poly(dA-dT). Biopolymers. 1985 Dec;24(12):2185–2194. doi: 10.1002/bip.360241203. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Bustamante C., Maestre M. F. The optical activity of nucleic acids and their aggregates. Annu Rev Biophys Bioeng. 1980;9:107–141. doi: 10.1146/annurev.bb.09.060180.000543. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Barton J. K., Magliozzo C. C., Tucker D., Lafer E. M., Stollar B. D. Lack of Z-DNA conformation in mitomycin-modified polynucleotides having inverted circular dichroism. Proc Natl Acad Sci U S A. 1983 May;80(10):2874–2878. doi: 10.1073/pnas.80.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Kleinwächter V., Palecek E. Salt-induced conformational changes of poly(dA-dT). Nucleic Acids Res. 1980 Sep 11;8(17):3965–3973. doi: 10.1093/nar/8.17.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Sklenár V. Salt-induced conformational transition of poly[d(A-T)] X poly[d(A-T)]. J Mol Biol. 1983 May 5;166(1):85–92. doi: 10.1016/s0022-2836(83)80052-7. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Larson J. E., Klysik J., Stirdivant S. M., Wells R. D. Conditions which cause the right-handed to left-handed DNA conformational transitions. Evidence for several types of left-handed DNA structures in solution. J Biol Chem. 1982 Mar 25;257(6):2775–2782. [PubMed] [Google Scholar]


