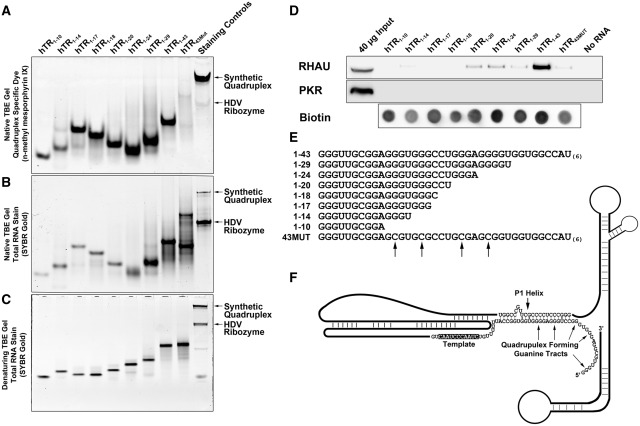
Figure 2.
hTR1–17 is the minimal sequence capable of forming a G4-quadruplex and hTR1–20 is the smallest truncation that demonstrates affinity for endogenous RHAU. (A) Approximately 200 pmols of each hTR RNA truncation was separated by native TBE polyacrylamide gel electrophoresis and stained with 1 µg/ml n-methyl mesoporphyrin IX diluted in 20 mM Tris pH 7.5, 100 mM KCl and 1 mM EDTA for 15 min at room temperature. The gel was imaged on a Fluorchem Q system using the Cy3 excitation and emission filters. (B) Approximately 5 pmols of each hTR RNA truncation was separated as in (A) and the gel was stained with the total RNA stain SYBR Gold according to the manufacturer’s protocol. (C) Approximately 5 pmols of each hTR RNA truncation was heated at 95°C for 5 min in 1× denaturing load dye and separated by denaturing TBE polyacrylamide gel electrophoresis and stained with the total RNA stain SYBR Gold according to the manufacturer’s protocol. (D) Western blot of proteins enriched by streptavidin pull-down assays performed with biotinylated hTR truncations. 3′ biotinylated hTR truncations were incubated with HEK293T whole cell extracts for 30 min and protein/RNA complexes were pulled-down with 50 µl streptavidin agarose beads. The beads were boiled for 5 min in 1× SDS loading dye and the binding of RHAU to each RNA was assessed by performing SDS/PAGE and western blotting. As a control for binding specificity, blots were reprobed with anti-PKR antibodies and to control for non-specific interactions with the streptavidin agarose, a beads alone (no RNA) control was performed. Biotinylation efficiency for each RNA is demonstrated by a dot blot of 5 pmols of each RNA detected with streptavidin-HRP. (E) Sequences of each of the RNAs used for the streptavidin pull-down assay. G to C substitutions made in 43MUT are indicated by arrows. (F) Schematic of the hTR RNA based upon the published proposed secondary structure highlighting the P1 helix and overlapping quadruplex forming guanine tracts (26).