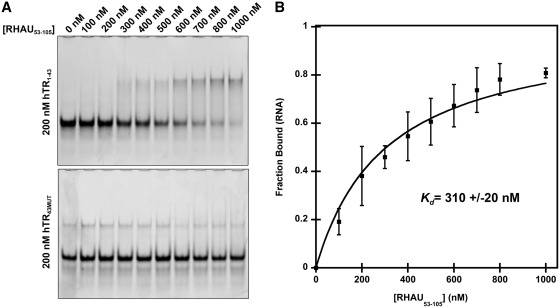
Figure 3.
hTR1–43 interacts with an N-terminal RHAU truncation (RHAU53–105) containing the RSM. (A) Electrophoretic mobility shift assay demonstrating a specific interaction of RHAU53–105 with hTR1–43. 200 nM hTR RNA was incubated in a binding reaction with increasing concentrations of RHAU53–105 for 15 min at room temperature and the free RNA and RNA/protein complexes were resolved by native TBE polyacrylamide gel electrophoresis and stained with the nucleic acid dye SYBR Gold. The lower gel demonstrates a loss of interaction when G to C substitutions are introduced into the RNA sequence (hTR43MUT). (B) Quantification of the free RNA band for hTR1–43. Data represent the mean of three independent experiments ± standard deviation. Curve fitting and calculation of Kd was performed by a previously published method (34). Additional gel images are provided in Supplementary Figure S1.