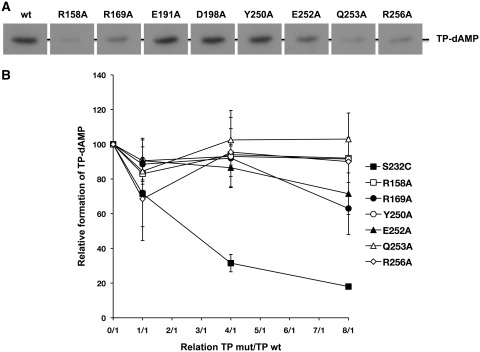
Figure 2.
(A) In vitro protein-primed initiation with TP mutants. The initiation assay was performed as described in ‘Materials and Methods’ section, in the presence of 10 ng of either wild-type or the indicated mutant TP, 20 ng of DNA polymerase and 500 ng of ϕ29 TP-DNA. After 4 min incubation at 30°C, the reactions were stopped, processed and analysed by SDS–PAGE and autoradiography. The position of the TP–dAMP complex is indicated. (B) Competition for DNA polymerase between wild-type and mutant TPs. The assay of formation of TP-dAMP in the absence of TP-DNA by the wild-type ϕ29 TP/DNA polymerase heterodimer was performed in the presence of increasing amounts of TP mutants (the reaction conditions are described under ‘Materials and Methods’ section). Reactions were started by adding 1 mM MnCl2 and, after incubation for 90 min at 30°C, were stopped and analysed as indicated for the protein-primed initiation assay. The TP-dAMP formed in the different competition conditions relative to that formed in the absence of competition (100%) is indicated. As control of competition, the inactive TP mutant S232C was used. Data are represented as mean ± SD corresponding to three independent experiments.