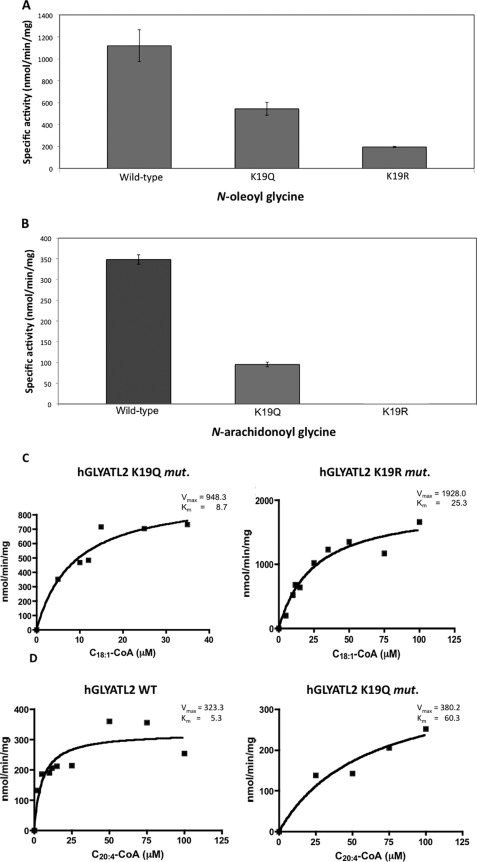
FIGURE 5.
Lysine 19 is an important residue in regulation of hGLYATL2 enzyme activity. A, ∼1 μg of hGLYATL2 (wild type), K19Q, and K19R mutant proteins was incubated with 50 μm C18:1-CoA, 50 μm C20:4-CoA, 50 mm glycine with the addition of BSA in a molar ratio of 1:5.5 BSA:acyl-CoA as outlined under “Experimental Procedures.” A, N-oleoyl glycine conjugates formed. B, N-arachidonoyl glycine conjugates formed and were quantified using ESI-MS. The experiment was repeated four times (three times for the K19Q mutant), and the mean ± S.D. is shown. C, recombinant K19Q and K19R proteins (∼1 μg) were incubated for 2 min at various concentrations of oleoyl-CoA (5–100 μm) (C) or N-arachidonoyl-CoA (5–100 μm) (D) with the addition of BSA in a molar ratio of 1:5.5 BSA:acyl-CoA in the presence of glycine (50 mm). N-Arachidonoyl glycine was added as an internal standard (5 μm) to reactions where N-oleoyl glycine is the product formed, and N-oleoyl glycine was added as an internal standard (5 μm) to reactions where N-arachidonoyl glycine is the product formed. Samples were purified on Evolute columns, analyzed by ESI-MS, and quantified according to the internal standard. Km (μm) and Vmax (nmol/min/mg) were calculated using Sigma Plot Enzyme Kinetics program. The experiments were repeated twice, and one representative experiment is shown.