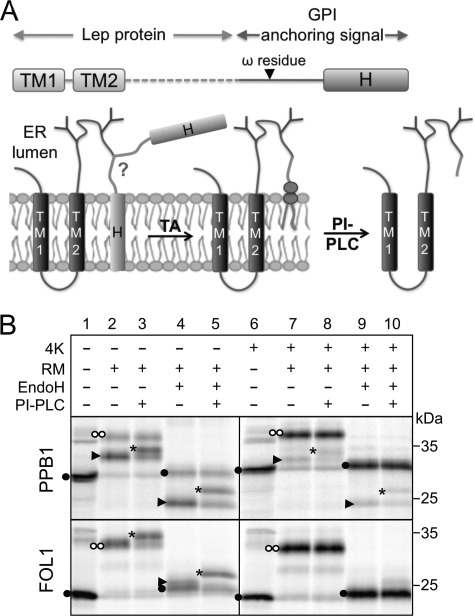
FIGURE 2.
A, experimental setup used in this study. Leader peptidase (Lep) fusion proteins bearing C-terminal GPI-anchoring signals are transcribed and translated in vitro in the presence of RM, where the proprotein becomes cleaved by the transamidase (TA) at the ω residue and anchored to a GPI lipid. PI-PLC can be used to remove the GPI lipid after solubilization of the microsomes. B, fusions of Lep with PPB1 and FOL1 signal sequences are efficiently GPI anchored. The GPI-anchored protein can migrate faster (PPB1, arrowhead, lane 2, upper panel) or similarly (FOL1) to the full-length protein (two white dots, lane 2, lower panel). GPI anchoring can in both cases be assayed for by treatment with PI-PLC, which results in a slower electrophoretic mobility (asterisk, lane 3). Because all constructs had two N-glycosylatable motifs, protein that has not been translocated into the microsomes can be identified as a low molecular weight band (black dot). Addition of 4 lysines (4K) at the C terminus greatly inhibits GPI anchoring of both constructs (lanes 6–10).