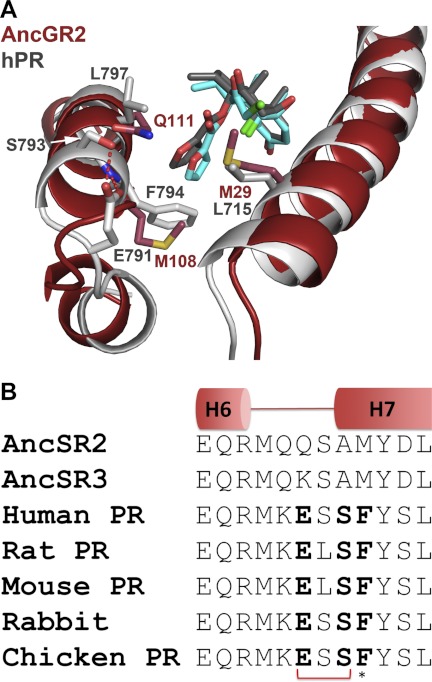
FIGURE 2.
Structural basis for off-target activation of PR. A, the human PR (hPR)-MOF complex (white; Protein Data Bank code 1SR7) superimposed on the AncGR2-MOF complex (red). PR residue Phe-794 maintains space for the 17α-furoate moiety and contributes a hydrophobic interaction via the aromatized side chain (15). PR residues 791ESSF794 on H7 appear to play a key role in allowing strong MOF binding by positioning the H6-H7 loop and H7 via a conserved Glu-791–Ser-793 H-bond. B, this motif is strictly conserved among extant PRs but is not present in AncSR2.