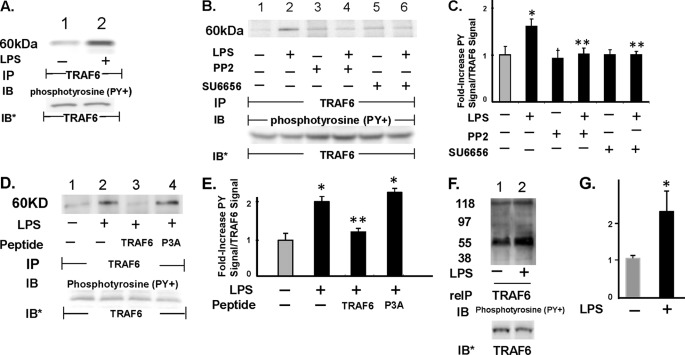
FIGURE 8.
SFK(s) increases tyrosine phosphorylation of TRAF6. A, HMVEC-Ls were treated for 0.5 h with LPS (300 ng/ml) or medium alone, and the cell lysates were immunoprecipitated with anti-TRAF6 antibodies. The TRAF6 immunoprecipitates were processed for phosphotyrosine immunoblotting. B, HMVEC-Ls preincubated with either PP2 or SU6656 were similarly treated with LPS or medium alone, after which they were lysed, and the lysates were processed for TRAF6 immunoprecipitation followed by phosphotyrosine immunoblotting. C, for each immunoblot generated in B, densitometric quantitation of each Tyr(P)-TRAF6 signal was normalized to total TRAF6 signal in the same lane in the same blot. Vertical bars represent mean (±S.E.) arbitrary densitometry units of Tyr(P)-TRAF6 signal normalized to arbitrary densitometry units of total TRAF6 signal (n ≥4). D, HMVEC-Ls were preincubated with cell-permeable TRAF6 decoy peptide or the P3A control peptide, after which they were exposed for 15 min to LPS (300 ng/ml) or medium alone and lysed, and the lysates were immunoprecipitated with anti-TRAF6 antibodies. The TRAF6 immunoprecipitates were processed for phosphotyrosine immunoblotting. E, for each immunoblot generated in D, densitometric quantitation of each phospho-TRAF6 signal was normalized to total TRAF6 signal in the same lane in the same blot. Vertical bars represent mean (±S.E.) arbitrary densitometry units of phospho-TRAF6 signal normalized to arbitrary densitometry units of total TRAF6 signal (n = 3). F, lysates of LPS-treated and medium control HMVEC-Ls were immunoprecipitated with anti-TRAF6 antibodies after which the immune complexes were resuspended in 2% SDS, heat-treated, and again immunoprecipitated with the same anti-TRAF6 antibody, and the TRAF6 immunoprecipitates were processed for phosphotyrosine immunoblotting. G, for each immunoblot generated in F, densitometric quantitation of each Tyr(P)-TRAF6 signal was normalized to total TRAF6 signal in the same lane in the same blot. Vertical bars represent mean (±S.E.) arbitrary densitometry units of Tyr(P)-TRAF6 signal normalized to arbitrary densitometry units of total TRAF6 signal (n = 3). *, significantly increased compared with the simultaneous medium control at p < 0.05. **, significantly decreased compared with the LPS-treated HMVEC-Ls at p < 0.05. A, B, D, and F, to control for efficiency of immunoprecipitation and protein loading and transfer, blots were stripped and reprobed with the immunoprecipitating antibody. IP, immunoprecipitate; reIP, reimmunoprecipitated; IB, immunoblot; IB*, immunoblot after stripping and reprobe. Molecular mass in kDa is indicated on left. Each blot is representative of ≥3 independent experiments.