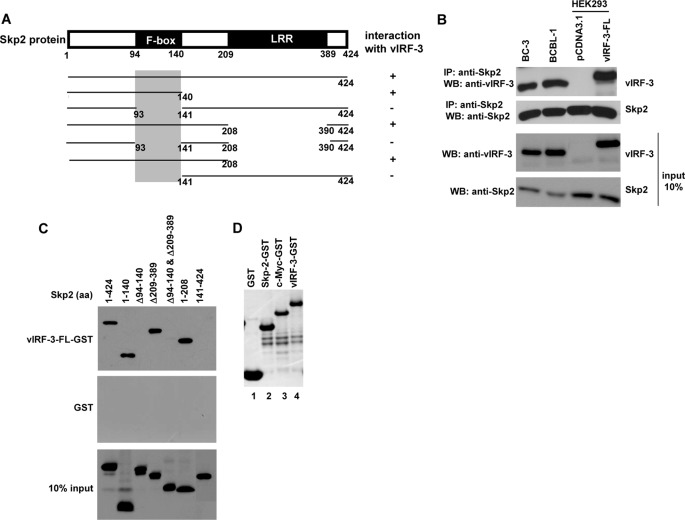
FIGURE 4.
vIRF-3 interacts with F-box of Skp2. A, schematic representation of Skp2 deletion mutants tagged with Myc tag. The shaded box indicates the vIRF-3-binding domain. LRR, leucine-rich region. B, co-immunoprecipitation of Skp2 and vIRF-3 in PEL cells, BC-3 and BCBL-1, and transfected HEK293 cells. Protein lysates (400 μg) were immunoprecipitated (IP) with anti-Skp2 antibodies, and the immunoprecipitated complexes were analyzed by Western blot (WB) with anti-vIRF-3 antibodies. The relative levels of Skp2 and vIRF-3 in 40 μg of protein lysates are shown for comparison (input 10 %). The higher molecular weight of transfected vIRF-3-FL is due to Myc-His tag at the carboxyl terminus. C, Myc-tagged skp2 deletion mutants were in vitro translated using the TnT T7 quick coupled transcription/translation system and incubated with vIRF-3-FL fused to GST or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with anti-Myc (9E10) antibodies. 10% of Skp2 deletion mutant protein input is shown below (10% input). aa, amino acids. D, Coomassie Blue staining of purified GST, Skp2-GST, c-Myc-GST, and vIRF-3-GST used in GST pulldown experiments.