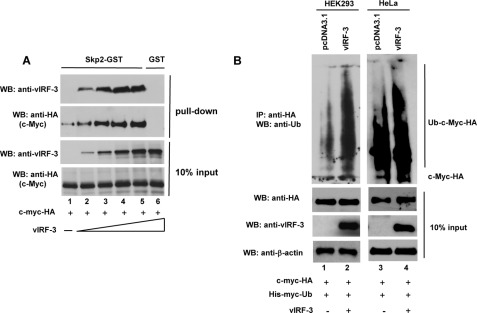
FIGURE 6.
vIRF-3 neither inhibits c-Myc/Skp2 interaction nor blocks c-Myc ubiquitylation. A, increased binding of c-Myc to Skp2 in the presence of vIRF-3. Constant amounts of in vitro translated c-Myc-HA protein were incubated with Skp2-GST immobilized to glutathione-Sepharose beads in the presence of increasing amounts of in vitro translated vIRF-3. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot (WB) with anti-HA (c-Myc) and anti-vIRF-3 antibodies. The binding to GST beads represents negative control (lane 6). 10% of c-Myc and vIRF-3 protein input is shown below (10% input). B, increased ubiquitylation of c-Myc in vIRF-3-expressing cells. HEK293 (lanes 1 and 2) and HeLa (lanes 3 and 4) cells were co-transfected with equal amounts of c-Myc-HA and His-Myc-Ub followed by transfection with either an empty vector (pcDNA3.1) or the vIRF-3-expressing construct. Prior to lysis, the cells were pretreated with proteasome inhibitor, 50 μm MG115, for 3 h. The cell lysates (400 μg) were analyzed by immunoprecipitation (IP) with anti-HA-specific antibodies followed by Western blotting with anti-Ub antibodies. The relative levels of transfected c-Myc-HA and vIRF-3 in 40 μg of cell lysates were estimated by Western blots (10% input).