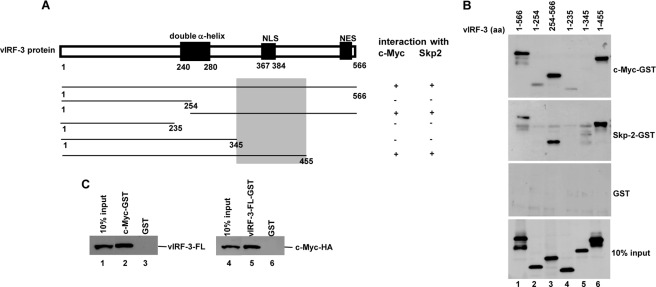
FIGURE 8.
c-Myc and Skp2 interact with vIRF-3 (amino acids 346–455). A, schematic representation of vIRF-3 N- and C-terminal deletion mutants. The shaded box indicates the common domain for c-Myc and Skp2 binding. NLS, nuclear localization signal; NES, nuclear export signal. B, vIRF-3 deletion mutants were in vitro translated using TnT T7 quick coupled transcription/translation system and incubated with c-Myc or Skp2 fused to GST or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with anti-vIRF-3 antibodies. 10% of vIRF-3 deletion mutant protein input is shown below (10% input). aa, amino acids. C, vIRF-3 (left panel) or c-Myc-HA (right panel) was in vitro translated using E. coli T7 S30 extract system and incubated with c-Myc-GST or vIRF-3-FL-GST, respectively. Incubation with GST alone served as a negative control. The bound proteins were eluted and resolved on 10% SDS-PAGE followed by Western blot with either anti-vIRF-3 or anti-HA antibodies. 10% of in vitro translated vIRF-3 or c-Myc-HA is also shown (10% input).