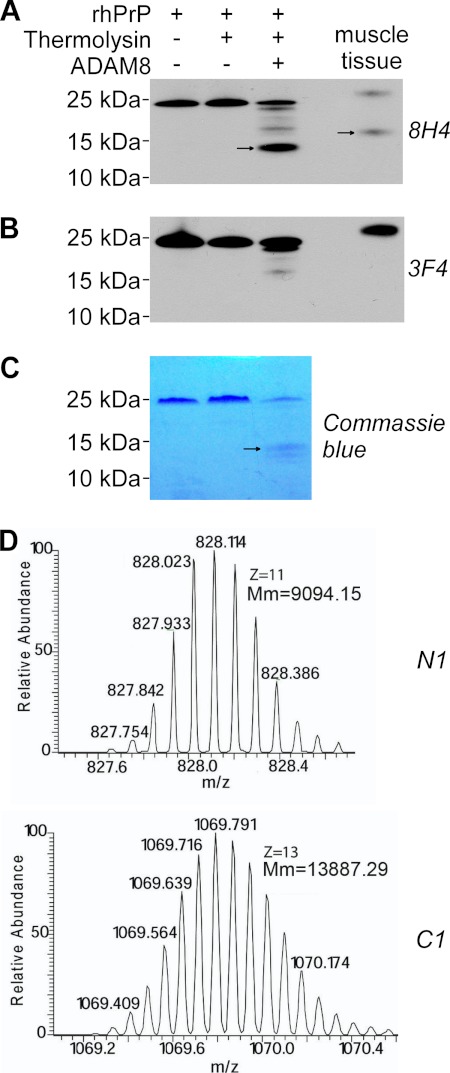
FIGURE 8.
ADAM8 directly cleaves PrP to generate the C1 fragment. Recombinant human PrP (rhPrP) was cleaved at 37 °C for 14 h with a recombinant human ADAM8 that had been treated or untreated with thermolysin for activation, followed by SDS-PAGE and subsequent immunoblot analysis (A and B) or direct staining with Coomassie Blue (C), or by direct mass spectrometry analysis (D). A, Western blot probed with antibodies 8H4 (against human PrP176–186). B, Western blot probed with 3F4 (against human PrP 106–112). C, SDS-PAGE gel directly stained with Coomassie Blue. D, mass spectrometry analysis. An in vitro reaction sample with thermolysin-activated ADAM8 and rhPrP was analyzed. Monoisotopic distributions of protonated (M+11H)11+ N1 fragment ion (top) and protonated (M+13H)13+ C1 fragment ion (bottom) are shown; Mm: molecular mass. In panels A-B, skeletal muscle tissue homogenate from a Tg(HQK) mouse treated with Dox for 60 days was loaded as a control. In panels A–C, the arrows point to the C1 band. The rhPrP sample contained an extension of Gly-Ser at the N terminus as a leftover from the His tag; accordingly, the resulting N1 fragment also contained the extra Gly-Ser residues at the N terminus.