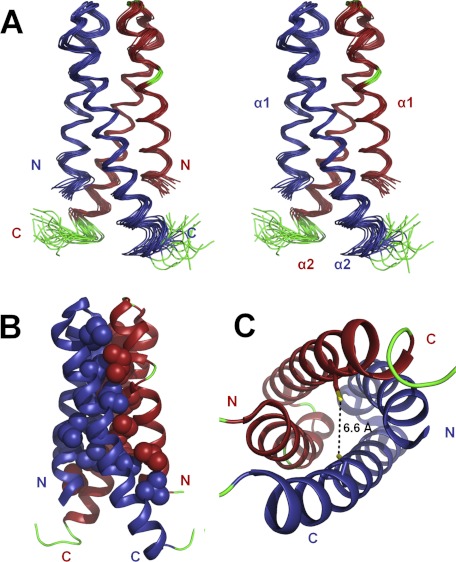
FIGURE 4.
Solution NMR structure of CDK2AP1(61–115). A, ensemble of the 20 lowest energy NMR-derived conformers in stereoview. B, lowest energy conformer showing hydrophobic side chains that stabilize the interface in a space-filling representation. C, top-down view of the four-helix dimeric structure with the side chains of the Cys-105 residues shown in stick representation. The thiol groups of the two Cys-105 residues are nearby one another in the three-dimensional structure and poised for disulfide bonding. In A–C, α-helices are shown in blue (chain 1) and red (chain 2), the loops and not well defined N- and C-terminal regions are shown in green, and the sulfur atoms of the Cys-105 residues are shown in yellow.