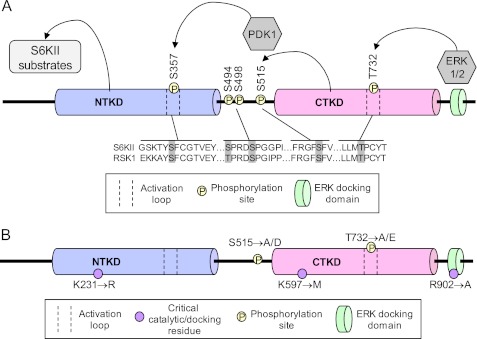
FIGURE 1.
S6KII domains and phosphorylation sites that may play a role in its circadian function. A, composition of the fly S6KII protein. Two kinase domains, the N-terminal kinase domain (NTKD) and the C-terminal kinase domain (CTKD), are joined by a linker region. S6KII also contains an ERK1/2-binding domain at its C terminus. Schematic depicts the series of events thought to lead to S6KII kinase activation (right to left), beginning with ERK binding to the C terminus and phosphorylation of T732. Arrows indicate phosphorylation events. Homology between S6KII and human RSK1 phosphorylation sites is shown. B, point mutations generated to disrupt N-terminal kinase activity (lysine, K to arginine, R), C-terminal kinase activity (lysine, K to methionine, M) and ERK-binding (arginine, R to alanine, A) are shown below the S6KII protein schematic. Serine and threonine phosphorylation sites mutated to be pseudophosphorylated (aspartate, D or glutamate, E) or unphosphorylatable (alanine, A) are depicted above the schematic.