Abstract
Background
Aspergillus flavus infection and aflatoxin contamination of maize pose negative impacts in agriculture and health. Commercial maize hybrids are generally susceptible to this fungus. Significant levels of host plant resistance have been observed in certain maize inbred lines. This study was conducted to identify maize genes associated with host plant resistance or susceptibility to A. flavus infection and aflatoxin accumulation.
Results
Genome wide gene expression levels with or without A. flavus inoculation were compared in two resistant maize inbred lines (Mp313E and Mp04∶86) in contrast to two susceptible maize inbred lines (Va35 and B73) by microarray analysis. Principal component analysis (PCA) was used to find genes contributing to the larger variances associated with the resistant or susceptible maize inbred lines. The significance levels of gene expression were determined by using SAS and LIMMA programs. Fifty candidate genes were selected and further investigated by quantitative RT-PCR (qRT-PCR) in a time-course study on Mp313E and Va35. Sixteen of the candidate genes were found to be highly expressed in Mp313E and fifteen in Va35. Out of the 31 highly expressed genes, eight were mapped to seven previously identified quantitative trait locus (QTL) regions. A gene encoding glycine-rich RNA binding protein 2 was found to be associated with the host hypersensitivity and susceptibility in Va35. A nuclear pore complex protein YUP85-like gene was found to be involved in the host resistance in Mp313E.
Conclusion
Maize genes associated with host plant resistance or susceptibility were identified by a combination of microarray analysis, qRT-PCR analysis, and QTL mapping methods. Our findings suggest that multiple mechanisms are involved in maize host plant defense systems in response to Aspergillus flavus infection and aflatoxin accumulation. These findings will be important in identification of DNA markers for breeding maize lines resistant to aflatoxin accumulation.
Introduction
The pathogenic fungus Aspergillus flavus draws considerable attention in agriculture because it produces aflatoxins that contaminate maize and other oilseed crops. Consumption of aflatoxin contaminated crops has been linked to liver cancer in animals and humans [1]. The U. S. Food and Drug Administration has set strict standards and regulations to control interstate commerce of aflatoxin contaminated feed and other products [2]. Aflatoxins are a group of polyketide-derived mycotoxins produced by toxigenic isolates of Aspergillus flavus and some other Aspergillus species upon colonization of host plants [3]. The fungal pathogenicity and the interactions between Aspergillus flavus and the host plant defense systems have been extensively studied in an effort to improve plant resistance and to reduce aflatoxin contamination [3], [4].
Plants respond to fungal pathogens through various defense mechanisms. The fungal pathogens are basically classified into groups of biotrophs, necrotrophs and hemibiotrophs [5]. The well studied gene-for-gene resistance system was usually observed in the interactions between biotrophic fungal pathogens and their host plants [5]. Upon such fungal infection, host plant resistance (R) proteins recognize the race-specific fungal elicitors and hence trigger a cascade of salicylate-dependent signal transduction pathways. As a result, the resistance reaction called hypersensitive response (HR) (localized lesion) takes place to limit fungal growth [6]–[8]. In contrast to the biotrophs, much less is known about the plant defense mechanism towards the necrotrophs. The plant resistance to necrotrophic fungal pathogens is likely controlled by quantitative resistance genes. Jasmonate- and ethylene-dependent signaling pathways are likely involved in such resistance systems. And they are triggered by fungal toxins or other fungal effectors [5]. Few resistance genes have been identified, and no gene-for-gene resistance systems have been reported in the necrotrophic fungal plant interactions [5]. Moreover, studies showed that the HR reactions actually enhance the host plant susceptibility and facilitate the necrotrophic fungal colonization [9]. Recently, a few structurally resistance-like (R-like) genes have been characterized to recognize fungal toxins and confer plant susceptibility during the necrotrophic fungal colonization (gene-for-gene susceptibility) [10], [11]. In such a case, host plant resistance exhibits in the form of insensitivity to fungal effectors in the plants that carry the mutant forms of the susceptibility-related R-like genes.
The toxigenic isolates of Aspergillus flavus possess the characteristics of necrotrophic fungal pathogens. Commercial maize hybrids are generally susceptible to Aspergillus flavus. The maize host resistance to Aspergillus flavus infection and aflatoxin accumulation has been identified in certain maize germplasm lines [12], [13]. Kernels of resistant maize lines show significantly less aflatoxin accumulation than susceptible maize lines at maturity. Previous studies on mapping populations derived from crosses between a resistant maize inbred line and a susceptible maize inbred line have led to the identification of several resistance-related quantitative trait loci (QTLs) in maize [14]–[16]. Most interestingly, QTLs for resistance to aflatoxin accumulation appeared to be derived from both resistant and susceptible parental lines (Willcox et al. 2000, unpublished data). Nevertheless, the molecular mechanisms underlying the significant aflatoxin reduction in resistant maize lines or the significant aflatoxin accumulation in susceptible maize lines are yet to be determined. To characterize maize genes involved in such host plant responses under Aspergillus flavus infection, we conducted a microarray analysis on kernel samples collected from a 4×2 factorial field experiment with two resistant maize inbred lines (Mp313E, Mp04∶86) in contrast to two susceptible inbred lines (Va35, B73). Candidate genes selected from the microarray analysis were further investigated by quantitative RT-PCR analysis between one resistant maize line (Mp313E) and one susceptible maize line (Va35) in a time course experiment.
Results
Statistical Analysis Revealed Gene Expression Patterns Specific to Resistant or Susceptible Maize Inbred Lines
Four maize inbred lines were used in this experiment. The field experimental design for all the maize inbred lines was a randomized complete block with split plot and three replications for each genotype. Table 1 shows comparison of the mean aflatoxin accumulation levels measured in mature maize kernels from the inoculated and un-inoculated primary ears of each genotype. Mp313E is a maize inbred line showing stable and inheritable resistance to Aspergillus flavus infection and low amount of aflatoxin accumulation in the kernels (Table 1). Va35 is a maize inbred line with good agronomic traits, but it shows susceptibility to Aspergillus flavus and high amounts of aflatoxin accumulation (Table 1). Mp04∶86 is a recombinant inbred line derived from a cross of maize lines Va35 (susceptible) and Mp715 (resistant). Thus Mp04∶86 shares genes from Va35, but it was selected for the trait of resistance to Aspergillus flavus and aflatoxin accumulation. B73 is an elite maize inbred line and a model resource for maize genome sequence information [17]. B73 is susceptible to Aspergillus flavus and also shows high amounts of aflatoxin accumulation (Table 1).
Table 1. Kernel aflatoxin levels in the four maize inbred lines used for the DNA microarray analysis.
| Pedigree | Host Plant Response | Treatment | Aflatoxin(ng/g) |
| Mp04∶86 | Resistant | Inoculated | 195 |
| Uninoculated | ∼ 1 | ||
| Va35 | Susceptible | Inoculated | 1243 |
| Uninoculated | ∼ 1 | ||
| Mp313E | Resistant | Inoculated | 140 |
| Uninoculated | ∼ 1 | ||
| B73 | Susceptible | Inoculated | 3791 |
| Uninoculated | ∼ 1 |
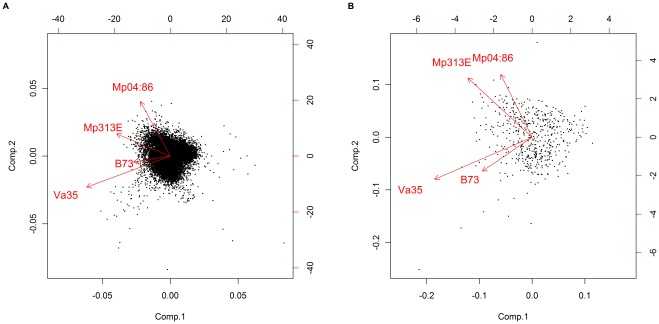
The maize oligonucleotide arrays (from NSF Maize Oligonucleotide Array Project) used in this experiment contained 57,452 maize gene probes. In our study, each microarray slide was hybridized with two samples (dual channel hybridization) that were the inoculated and uninoculated samples from the same genotype and the same plot. In such an array design we first evaluated the resistance and susceptibility responses in each genotype and then compared the differentially expressed genes between groups of two resistant and two susceptible maize inbred lines. We performed multiple analyses on the microarray data using different statistical algorithms to reveal the host plant specific responses to Aspergillus flavus infection and aflatoxin reduction. We first analyzed microarray data with SAS Version 9.1.3 [18] using the mixed model for a split plot design with four maize inbred lines as main unit and two treatments [inoculated (I) vs. uninoculated (U)] as subunit. Log transformed median expression values were used to obtain estimates of gene expression ratios (I/U) for each genotype. Then the log2 values of the expression ratios (I/U) of 13,107 expressed genes from all genotypes were analyzed by principal component analysis (PCA). Figure 1A and 1B are biplots showing the distribution of the log2 gene expression ratios (I/U) on a projected principal plane. The data appeared to be cloudy, however, we found that the vectors representing the larger variances associated with the two resistant maize inbred lines (Mp313E and Mp04∶86) were clustered together. Likewise, the vectors representing the larger variances associated with the two susceptible maize inbred lines (Va35 and B73) were clustered together. That means despite the differences in the genomes of these genotypes, trends of gene expression associated with host plant resistance or susceptibility were evident. For example, Mp04∶86 showed a distinguished expression pattern compared to its susceptible parent Va35 in response to the fungal infection (Figure 1A and 1B). The PCA analysis has revealed a separation of genes between the resistant and susceptible groups in response to Aspergillus flavus colonization. Genes that expressed toward the larger variances were considered as associated with the corresponding traits. Genes expressed toward the resistance trait located close to the vectors for Mp313E and Mp04∶86. Genes for susceptibility located close to the vectors for Va35 and B73 (Figure 1A and 1B). By this method, we grouped genes for possible candidates contributing to either the host resistance or the host susceptibility.
Figure 1. Biplots showing results of the principal component analysis (PCA) on log2 expression ratios (I/U) of 13,107 expressed genes.
The distribution of the gene expression values shows evident trends represented by the vectors associated with resistant and susceptible maize inbred lines. Genes expressed toward the resistance trait were located close to the vectors for Mp313E and Mp04∶86. Genes contributing to susceptibility were located close to the vectors for Va35 and B73. 1A. Biplot of principal component analysis (PCA) on 13,107 expressed gene probes. 1B. Biplot of principal component analysis (PCA) on a subset of 500 expressed gene probes. (The arrows represent vectors. The direction and length represent the larger variance of the expression values).
As a computational validation step and an exploratory method to look at effects of different algorithms on test of the significance levels, we analyzed the microarray data using the R package of the Linear Models for Microarray Data (LIMMA) [19]. LIMMA uses Bayes method which is a different algorithm for identification of significantly expressed genes. Both SAS and LIMMA methods yielded large number of differentially expressed genes. To screen for the genes that have larger impacts on the traits and to reduce the number of less relevant genes, the lists of top ranked genes from LIMMA analysis were compared with the lists obtained from the analysis with SAS. Genes significant (P<0.01) from the LIMMA lists and ranking high on the SAS lists were selected. Final candidate genes for further quantitative analysis with qRT-PCR were selected by integration of the results from PCA, LIMMA and SAS analysis.
Time Course Quantitative Expression Analysis on Selected Candidate Genes
The maize microarray analysis provided a list of candidate genes possibly contributing to the host plant resistance and susceptibility. To verify and study the selected candidate genes in more details, we conducted a separate experiment for a time course quantitative study using qRT-PCR method. The resistant maize inbred line Mp313E and the susceptible maize inbred line Va35 were selected for this study. The field experimental design was a randomized complete block design with three replications for each genotype. Kernel samples were collected in the field over eight time points. The first collection was performed within three hours after the fungal inoculation on the same day (0) and the other collecting time points were at 1, 2, 3, 4, 7, 14, and 21 days after inoculation. There were three replications collected for each time point. We prepared RNA samples and performed qRT-PCR analysis to evaluate the expression levels of 50 candidate genes (Table 2) in Mp313E and Va35. The maize GAPDH gene was used for normalization. The qRT-PCR data were analyzed using the software GenEx 5. 0. 1 [20]. Figure 2 is a bar-graph showing the comparison on the grand mean expression levels for each gene between Mp313E and Va35 lines. Thirty-one of the 50 genes were found to be significantly differentially expressed (P<0.05) by a paired t-test between the Mp313E and Va35 samples (Table 3). PCA analysis on the samples showed that Mp313E and Va35 samples were classified into two distinct groups (Figure 3), which indicated that the criteria we used for candidate gene selection from the microarray data were effective in reflecting host plant specific responses to the fungal infection. Figure 4 is a plot from a PCA analysis on the 50 candidate genes to visualize group of genes differentially expressed in Mp313E versus Va35. Genes highly expressed in Mp313E were present in the area with positive y axis coordinates in this plot, whereas genes located in the area with negative y axis coordinates in this plot were those expressed more inVa35 (Figure 4).
Table 2. Primer sequences of candidate genes and the house keeping gene GAPDH in qRT-PCR study.
| Primer ID | Primer Sequences |
| AI065864F | AGAATCGATCCGCCAAGTTA |
| AI065864R | AGGTTGCAACGCTATTGGTC |
| AI065909F | TACCACAGCAGAGCAACCAC |
| AI065909R | ATCTCCGGCTGAAGAAGACA |
| AI664980F | CTGACACAAAGCGACCTTCA |
| AI664980R | ATCCTGTTCGCTACCGTGTT |
| AI665626F | GGCACTGTCATCATGTTTGG |
| AI665626R | TATGATGCCTTCGACGATGA |
| AI857200F | GAAAATGACCCACCGAATTG |
| AI857200R | AGAGGAAGGACGCCCACTAT |
| AW017563F | TGTGCTCCGCTACTCAAATG |
| AW017563R | AACGGCCTAGATCCAATGTG |
| AW065862F | CGAGCGTCTTACAACAACCA |
| AW065862R | GGGTTCACCATGGCTAGGTA |
| AZM4_122338F | ATGGAACGAGGAGAAGAGCA |
| AZM4_122338R | TCCTGCACACACAAGAGTCC |
| BE050050F | CCGTGGAAATGTGGTAATCC |
| BE050050R | ATCCACGTCAACCATCTTCC |
| BG266083F | CTTTGCATCACAAAGCTCCA |
| BG266083R | GGTGAGGAAGAGCAAATGGT |
| BM078796F | TTTTCTCCACCTCGGTCTTG |
| BM078796R | AGCGTGAGCTCCTACGACAT |
| BM341348F | CTAGGAAACACCCGTCGGTA |
| BM341348R | CAATTGCTGCCATACAACCA |
| BM379345F | TTCACACACACCACACAATACC |
| BM379345R | CTGCAACTGTTGATCCCATC |
| BM498943F | CTCTGTATTGGCCCACGACT |
| BM498943R | AATTGTCGAGGTCGGAGATG |
| BQ538849F | TGATGAACAACCAAGCAAGAG |
| BQ538849R | ACATGGCAACGATACACGAA |
| BU036535F | ATCACTAATCTCACGCAACTCG |
| BU036535R | GCCCAAAGCTGTTGGATAGA |
| CA399536F | GGCTGATGCAATAAGGTGGT |
| CA399536R | TTGTTGCCATTCTACCCACA |
| CD433043F | TCTTCTTCCCCCGTACCTCT |
| CD433043R | AGGGCTGATGATTGTTGGAG |
| CD443591F | ATAGCAGCCATCCTCCATTG |
| CD443591R | GGGAAGAACATCCCCTTGAT |
| CD447259F | TCCTTGGACTTTCTGCGAGT |
| CD447259R | CATCACGAATACACCGTTGG |
| CD447608F | AAAAGGTAGCAGGGCTCACA |
| CD447608R | CCATTCAGCCGGTTATTTGT |
| CD448520F | GTGGGGCGATATTACTGCAT |
| CD448520R | GGGTTTCAACTGCCATTCC |
| CD448671F | GGATTGTCTTCCATGCAACC |
| CD448671R | GAAATTGCTGGGGGTAGTCA |
| CD987262F | ATGCGCGTACTTGCCTAAAC |
| CD987262R | TCAGGTACAACTCGCCCTTC |
| CF001049F | CTAAAAGAGGGCACCACCAA |
| CF001049R | TTGGCACCCTATTACAACTGC |
| CF007590F | GACGACGCCAGTATGTGATG |
| CF007590R | TAAACGAAGCTAGCGCACAA |
| MZ_GAPDHF | CGACTTACTTGGTGACAGCAG |
| MZ_GAPDHR | CGCCATCCACATTTATTCTCG |
| TC202886F | GAGCTTGGTGCTGGAATAGG |
| TC202886R | TCGCTTGAGCCTCTCTGAAT |
| TC207503F | AAACGCCATTGCACATTACA |
| TC207503R | TCTTGAAGGATCGTGTGCTG |
| TC218605F | AGGTTTTCGACAGCAGCAAT |
| TC218605R | GCCTCTTCACCAGCTAATGC |
| TC219510F | CTCCCTAGCCAACACACACA |
| TC219510R | GTCCCGGTGTAACAAACGAG |
| TC220132F | GTGGTCTTGACTGGGGTGTT |
| TC220132R | TCACAAAGCCAAAGCCTCTT |
| TC220895F | CAGCGAGATCAACAAGAGCA |
| TC220895R | GGCCCGTAGTTGTAGTTCCA |
| TC221540F | CAGCTGTGGCAGGACTACAA |
| TC221540R | TCATACCAAACGCATTGAGC |
| TC223372F | AGGATCTGGGGATGGATTTC |
| TC223372R | CATGCATGGTCGTGTTTTGT |
| TC223736F | AACGGTCAGAATTGGAGTGC |
| TC223736R | GACGACGCAACAGATCTCAA |
| TC225075F | CTGCTGATCGAGACATTGGA |
| TC225075R | AAGACATGCAACCAACACCA |
| TC226528F | TGTTCGTCCTCTGCTTGTTG |
| TC226528R | TAATGGGTGGAAGGAATGGA |
| TC227223F | CAGCCAAGATGTTTGCATTG |
| TC227223R | ATCCATGGGTTCATGGTAGC |
| TC227578F | AGCGTGAGCTCCTACGACAT |
| TC227578R | CAACCCACGCTAGTGCTACA |
| TC231674F | GGGCTTCTTGTTGTGCTCTC |
| TC231674R | TTAAAGCGCTGCCTTATTCC |
| TC234808F | AATGTCGACCTTGGAACTGC |
| TC234808R | CTGCAGGGGCTTCTTTACTG |
| TC235693F | GCAAGCTTCTGGTCATCCTC |
| TC235693R | TTCTCTGCAACAATGCCAAC |
| TC237311F | TGAGGATCATGGAGGAGGAC |
| TC237311R | CCACATTCACGGGCTTATCT |
| TC238832F | AGACATGGGATACCGAGACG |
| TC238832R | AGCTCCATCAGCTCCTTGAA |
| TC239720F | GATTCCTGATCCGAAGGACA |
| TC239720R | TTCCAAGGTCCCTTGTATGC |
| TC241201F | TCTTTCGACTGGTGATGCTG |
| TC241201R | CCCCACTGCATGTAGGACTT |
| TC241434F | ACAACCTCACCTTTGCAACC |
| TC241434R | GCTGCTATGTACGCCATCAA |
| TC245683F | CGGAATGGTACTCCTGGTTG |
| TC245683R | TGGGAGTCTCACACTCACGAT |
| TC247516F | AGGGCACCATGAGAAATCTG |
| TC247516F | AGGGCACCATGAGAAATCTG |
| TC247516R | GAATGCTGGTCCTGTTGGAT |
| TC247516R | GAATGCTGGTCCTGTTGGAT |
| TC247683F | ATGATGGGAGGCTGACTTTG |
| TC247683R | TCTCAGCGAAATTCATCGTG |
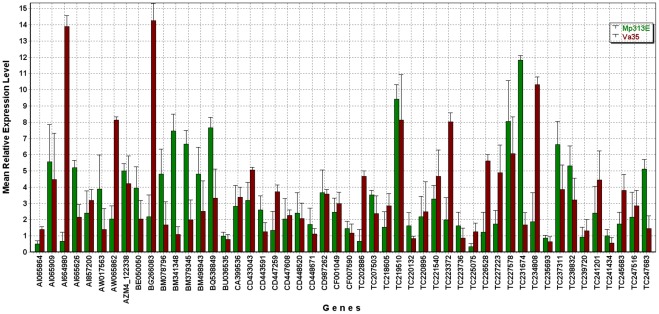
Figure 2. Bar-graph showing the comparison of the grand mean relative expression levels for each candidate gene in Mp313E and Va35 lines over all time points.
Thirty-one of the 50 genes were found to be significantly differentially expressed (P<0.05) by a paired t-test between the Mp313E and Va35 samples. The error bars represent 95% CI values.
Table 3. Genes differentially expressed in the resistant maize inbred line Mp313E and the susceptible maize inbred line Va35 verified by qRT-PCR method.
| Host Plant | ID | Chr | Bin | Function |
| Mp313E | BM078796*** | Chr 1 | 1.03 | Heat Shock Protein 26 (HSP26) |
| TC238832 ** | Chr 2 | 2.06 | Lecithin cholesterol acyltransferase (LCAT) | |
| BM498943 *** | Chr 3 | 3.08 | Ethylene Responsive Protein (ETHRP) | |
| BQ538849 ** | Chr 4 | 4.00 | C2H2-type Family Protein | |
| BM379345 *** | Chr 4 | 4.01 | Metallothionein-Like Protein (MTLP) | |
| BM341348*** | Chr 4 | 4.01 | Zein | |
| BE050050 * | Chr 4 | 4.05 | In Chr4 QTL | |
| TC223736 * | Chr 4 | 4.10 | Heat Shock Protein 90 (HSP90) | |
| TC241434 * | Chr 5 | 5.03 | ||
| TC231674 *** | Chr 5 | 5.05 | NPCs-NUP85, RNA Transport | |
| CD443591 * | Chr 6 | 6.04 | ||
| AI665626*** | Chr 6 | 6.05 | Zein | |
| TC237311* | Chr 6 | 6.07 | Heat Shock Protein (HSP 101) | |
| TC207503 * | Chr 8 | 8.03 | Prenylated Rab Acceptor (PRA1) Family Protein | |
| TC247683 *** | Chr 8 | 8.05 | ||
| AW017563* a | NA | NA | ||
| Va35 | AI664980 *** | Chr 1 | 1.06 | Glycine Rich RNA Binding Protein2(GRBP2), in Chr1 QTL |
| TC218605 * | Chr 1 | 1.09 | Phytochrome A (PHYA) | |
| TC241201* | Chr 2 | 2.07 | ||
| TC202886 *** | Chr 2 | 2.08 | ||
| TC226528 ** | Chr 3 | 3.05 | Uracil Permease (UPS) | |
| AW065862*** | Chr 4 | 4.01 | Zein | |
| CD433043 * | Chr 4 | 4.01 | Zein | |
| CD447259* | Chr 4 | 4.01 | Zein | |
| AI065864** | Chr 5 | 5.05 | Exonuclease-Endonuclease-Phosphatase (EEP) | |
| TC225075* | Chr 5 | 5.07 | Choline Transport | |
| TC245683 ** | Chr 6 | 6.02 | ||
| TC227223 * | Chr 6 | 6.05 | Zein | |
| TC223372 *** | Chr 7 | 7.02 | Cinnamoyl CoA Reductase (CNCR 2) | |
| TC234808 *** | Chr 8 | 8.01 | Ribosomal Protein L30 (RPL30) | |
| BG266083 *** | Chr 9 | 9.05 | HSP18a |
p value <0.05, **p value <0.001, ***p value <0.0001. The significance levels were determined by a paired t-test for the qRT-PCR data. a AW017563 chromosome and bin information is not available.
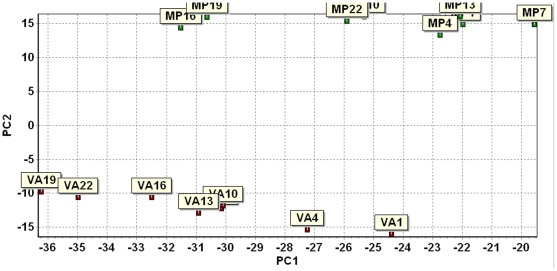
Figure 3. Plot showing the grouping of Mp313E and Va35 samples in the qRT-PCR study by principal component analysis.
Notice the Mp313E and Va35 samples were grouped into two distinct groups. It indicated the criteria used for candidate gene selection from the microarray data were effective in reflecting host plant specific responses to the fungal infection.
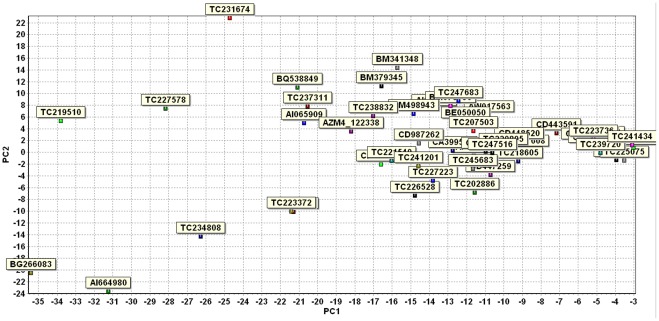
Figure 4. Plot of PCA analysis on the 50 candidate genes differentially expressed in Mp313E versus Va35.
Genes highly expressed in Mp313E were presented in the area with positive values of y axis coordinates, whereas genes located in the area with negative values of y axis coordinates were those expressed more inVa35.
Genes Highly Expressed in Resistant Maize Inbred Line Mp313E
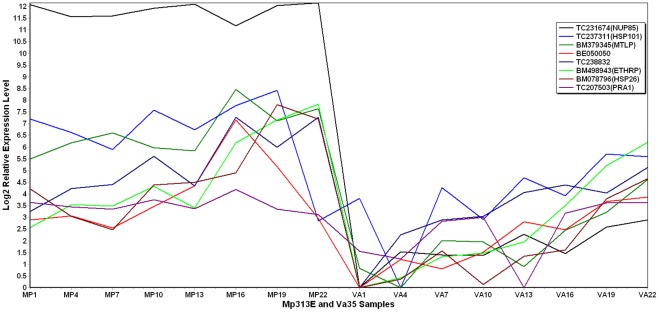
Sixteen of the 50 candidate genes showed significantly higher expression levels in the resistant maize inbred line Mp313E than in the susceptible line Va35 (Table 3). The gene functions were determined by searching the Genbank and the Maize GDB. Figure 5 is a 2-D plot showing quantitative comparison for some of the significant genes over a period of 21 days after inoculation with the fungus in Mp313E and Va35. TC231674 is the highest expressed found in Mp313E samples. TC231674 encodes a NUP85-like gene that is a part of a sub-complex of the nuclear pore complexes (NPCs) embedded in the nuclear envelope. The function of NPCs is for the transport of RNA and other macromolecules from nucleus to cytoplasm. TC237311 and BM379345 are second highest in expression. TC237311 encodes the heat shock protein HSP101. This protein acts as a molecular chaperone to disaggregate mis-folded proteins. BM379345 encodes a metallothionein like protein (MTLP) that is involved in the binding and detoxification of heavy metal ions. BE050050 and TC238832 comprise the next level in expression. BE050050 has no annotation available. TC238832 encodes a lecithin cholesterol acyltransferase (LCAT)-like gene. Other highly expressed genes include BM498943, BM078796, and TC207503. BM498943 encodes ethylene responsive protein (ETHRP) that belongs to the universal stress protein family. BM078796 encodes small heat shock protein HSP26. TC207503 encodes a prenylated rab acceptor (PRA1) family protein. Prenylated Rab PRA1 proteins are small transmembrane proteins that regulate vesicle trafficking.
Figure 5. 2D plot showing gene expression levels over a period of 21 days after the fungal inoculation.
These genes are highly expressed in Mp313E than in Va35.
Genes Significantly Expressed More in the Susceptible Maize Inbred Line Va35
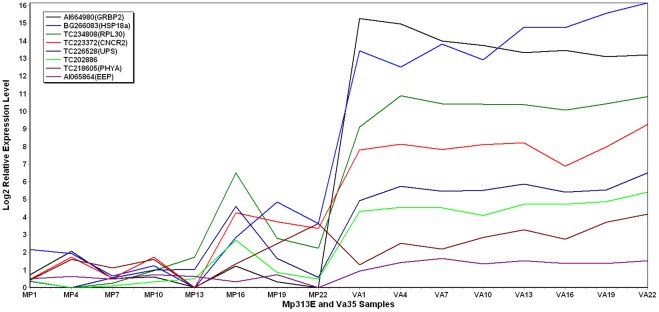
Fifteen genes were found to be significantly expressed more in the susceptible line Va35 than in the resistant line Mp313E (Table 3). The highest expressed genes found in Va35 were AI664980 and BG266083 (Figure 6). AI664980 belongs to the glycine-rich RNA binding protein family (GRBP2) that has RNA binding domains (RBD) commonly found in proteins involved in post-transcriptional gene expression processes. BG266083 encodes alpha-crystallin-type of stress-induced small heat shock proteins (HSP18a). TC234808 and TC223372 were also highly expressed in Va35. TC234808 is in the ribosomal protein L30 family (RPL30). The ribosomal protein L30 has pre-mRNA splicing regulatory activity to its own transcript and plays a key role in the assembling of the ribosomal subunits. TC223372 is a cinnamoyl-CoA reductase (CNCR2) that catalyses the lignin pathway. Other highly expressed genes found in Va35 included TC218605, TC226528, and AI065864. TC218605 encodes phytochrome A (PHYA). TC226528 encodes a ureide permease (UPS) that transport and recycle organic nitrogen for nucleotide synthesis. AI065864 belongs to the exonuclease-endonuclease-phosphatase (EEP) domain superfamily (Figure 6). There is no annotation for TC202886 in the database.
Figure 6. 2D plot showing gene expression levels over a period of 21 days after the fungal inoculation.
These genes are highly expressed in Va35 than in Mp313E.
Mapping of the Highly Expressed Genes to the QTL Maps
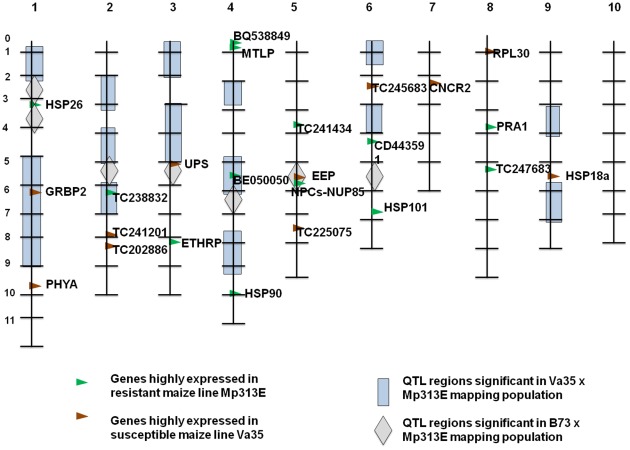
Figure 7 is a maize chromosome bin map showing the identified QTL regions from previous studies on two QTL mapping populations [14, 15, and Willcox et al. 2000, unpublished data]. We mapped the highly expressed candidate genes and the previously identified QTLs on the chromosome bin map to compare their relative chromosomal positions. Seven genes were mapped within and one gene close to the most significant QTL regions identified from the two mapping populations (Figure 7). The top highly expressed gene in Va35, AI664980 (Chr1, bin1.06), is located in the most significant chromosome 1 QTL region (Chr 1, bin 1.5–1.9) identified from the Va35 x Mp313E population. BG266083 (Chr 9, bin 9.05) is located close to the chromosome 9 QTL region (Chr 9, bin 9.06–9.07). The top highly expressed gene in Mp313E, TC231674 (Chr 5, bin 5.05), is mapped to the chromosome 5 QTL region (Chr 5, bin 5.05) identified in B73 x Mp313E population. BE050050 (Chr 4, bin 4.05) has no known function, but it is located within the chromosome 4 QTL region (Chr 4, 4.05–4.06) identified from both of the mapping populations. TC238832 (Chr 2, bin 2.06) is within a chromosome 2 QTL region (Chr 2, 2.06–2.07) from the Va35 x Mp313E population and close to a QTL region (Chr 2, bin 2.05) in the B73 x Mp313E population. BM078796 (HSP26) (Chr1, bin 1.03) is located in the chromosome 1 QTL region (Chr1, bin 1.03) in B73 x Mp313E population. These findings will be important in the identification of appropriate DNA markers for breeding of Aspergillus flavus and aflatoxin resistance maize lines.
Figure 7. Chromosome bin map showing the positions of the significant genes and the previously identified QTL regions.
Eight genes were mapped within or close to the seven most significant QTL regions. The top highly expressed gene in Va35, AI664980 (Chr1, bin1.06), is located in the most significant chromosome 1 QTL region (Chr 1, bin 1.5–1.9) identified from the Va35 x Mp313E population. The top highly expressed gene in Mp313E, TC231674 (Chr 5, bin 5.05), is mapped to the chromosome 5 QTL region (Chr 5, bin 5.05) identified from B73 x Mp313E population.
Discussion
Aspergillus flavus infection is a major concern for maize producers. Constant efforts are being made by plant breeders to develop resistant genotypes. Identification of genes having larger effects on the resistance or susceptibility is important to facilitate molecular marker-assisted breeding of resistant maize lines. Here, we evaluated different statistical tools for microarray data analysis, combined different methods to select the potentially relevant genes, and then conducted further quantitative expression analysis to take a closer look at the highly expressed host plant specific genes. Despite the complexity of the genotypic and environmental effects, the resistant and susceptible maize inbred lines exhibited distinguishable gene expression patterns in response to Aspergillus flavus colonization. These findings indicate that there are general maize host plant- Aspergillus flavus recognition and interaction processes that underlie resistance and susceptibility.
The susceptible maize inbred line Va35 showed hypersensitivity in response to Aspergillus flavus infection. Two highly expressed genes in Va35 are known to play roles in the plant responses toward various stress and pathogens. AI664980 encodes a glycine-rich RNA binding protein (GRBP2). A number of plant GRBPs were originally characterized as a result of cloning stress responsive genes. GRBPs were found to be associated with a variety of biotic and abiotic stresses including hormone, temperature, wounding, and pathogen infection [21], [22]. It was demonstrated that a tobacco GRBP gene was differentially expressed during HR reaction and it was also inducible by exogenous salicylic acid, suggesting that it plays roles in plant defense signal transduction and HR reaction [21]. GRBPs function through binding to RNA molecules and interfering with the post-transcriptional modifications. It has also been shown that GRBPs can interact with multiple proteins and RNA molecules, including its own mRNA, which is a feed-back mechanism for regulatory proteins [23]. TC234808 encodes a protein in the ribosomal protein L30 (RPL30) family. A RPL30 protein binds to its own transcript to inhibit the splicing and the translation of its own mRNA when it is expressed in excess. RPL30 proteins are important for governing the large and small ribosomal subunits assembling [24]. Both AI664980 and TC234808 genes have RNA binding domains and are likely involved in the post-transcriptional regulation of the genes in plant defense systems. BG266083 encodes alpha-crystallin-type of stress-induced small heat shock proteins (sHSPs) namely HSP18a. The sHSPs proteins are ubiquitous stress proteins that act as chaperones to prevent protein aggregations [25]. The roles of other highly expressed genes found in Va35 in response to Aspergillus flavus infection are not clear. It may be worth mentioning that TC226528 encodes a ureide permease (UPS) that transports and recycles organic nitrogen for nucleotide synthesis, indicating elevated activities in the metabolism of RNA molecules in Va35. AI065864 belongs to an exonuclease-endonuclease-phosphatase (EEP) domain superfamily. Enzymes in this large superfamily have the catalytic domain for cleaving phosphodiester bonds in nucleic acids. TC223372 encodes a cinnamoyl-CoA reductase (CNCR2) that catalyses the first step of the lignin pathway. The high level of TC223372 gene expression suggests that cell wall lignifications are probably involved in the infected Va35 plants.
The observed hypersensitive expression of stress responsive genes in Va35 appeared to be associated with its susceptibility to Aspergillus flavus. The hypersensitive response in Va35 likely causes cell death, and as a result, Aspergillus flavus infects and colonizes the host plant. In contrast, only very low level of AI664980 and TC234808 mRNAs were detected in the resistant maize inbred line Mp313E, which likely indicates that an insensitivity mechanism of the resistance is involved in Mp313E in response to the Aspergillus flavus infection. This hypothesis can explain some evidence found in the previous QTL studies. For example, AI664980 (Chr 1, bin 1.06) is located within the chromosome 1 QTL region (Chr 1, bins 1.05–1.09) identified in the Va35 x Mp313E population (Figure 7). This QTL was the most significant one and was identified from each of the planting locations and over all the three years (14, and Willcox et al. 2000 unpublished data). However, the resistance source of this QTL appeared to be associated with the susceptible Va35 genotype. Because this QTL had a big span and there were only a few polymorphic DNA markers available at the time, it is likely that there were crossovers that were not detected in that region, and the resistance was actually from the insensitive Mp313E allele. Similarly, it was possible for the reason of lacking suitable DNA markers that BG266083 (HSP18a) (Chr 9, bin 9.05) was mapped close to, but not within the chromosome 9 QTL (Chr 9, bin 9.06–9.07) region, and TC234808 (RPL30) (Chr 8, bin 8.01) was undetected in the previous QTL study. More information will come from an ongoing cloning and sequencing project for different alleles of these genes.
In addition to its apparent lacking of a sensitive form of the AI664980 allele, the resistant maize inbred line Mp313E appears to possess other resistance mechanisms as well. Evidence from our quantitative expression analysis showed that genes encoding RNA transport regulators, molecular chaperones, and detoxification proteins were highly expressed in Mp313E. The highest expressed gene TC231674 (Chr 5, bin 5.05) is homologous to the human nucleoporin NUP85 which is a component of the nuclear pore complexes (NPCs) in nuclear envelope. Nucleoporins are conserved from yeast to human. NPCs are involved in the transport of RNA and other macromolecules which is a fundamental regulatory process for plant defense system [26]. Recent studies have strongly suggested that components of NPCs regulate the transport of R proteins [26], [27]. A partial loss-of-function MOS7/NUP88 mutant gene in Arabidopsis was found to be associated with the suppression of the R protein snc1 mediated resistance [27]. In our study, we found that the NUP85-like nucleoporin gene was highly expressed in the resistant line Mp313E. In contrast, only low expression level of this gene was found in the susceptible line Va35. This indicates that the NPCs-related regulatory activities are involved in the maize host resistance associated with Mp313E. The highly expressed molecular chaperones in Mp313E included heat shock proteins HSP26, HSP90, and HSP 101. In addition, BM498943 encodes ethylene responsive protein (ETHRP) that belongs to the universal stress protein family. The universal stress proteins confer stress endurance and increase cell survival rate in general. TC207503 encodes a prenylated rab acceptor (PRA1) protein. Prenylated Rab PRA1 proteins are small transmembrane proteins that are involved in intracellular vesicle trafficking and the secretory pathways in cells. BM379345 encodes a metallothionein like protein (MTLP) that is involved in the binding and detoxification heavy metal ions. It was found that increased Aflatoxin production was associated with high levels of certain trace metal elements [28]. Evidence supporting the multiple resistance mechanisms present in Mp313E was also obtained from the previously conducted QTL studies in the B73 x Mp313E and Va35 x Mp313E populations. Two significant resistance QTLs were found to be associated with Mp313E genotype [14, 15, and Willcox et al. 2000, unpublished data], indicating that multiple defense systems are involved in Mp313E. Interestingly, the susceptible maize inbred line B73 appeared to have a different allele of AI664980 from Va35 (data not show), indicating that there are also multiple mechanisms underlying maize host susceptibility in response to Aspergillus flavus infection.
The resistance of maize to Aspergillus flavus infection and aflatoxin accumulation has been investigated from various aspects [29], [30]. However, this is the first comprehensive quantitative expression analysis to characterize genes associated with maize host plant hypersensitive responses and susceptibility in Va35, to identify genes that are associated with resistance in Mp313E, and to map quantitatively verified genes to previously identified major QTL regions. Our findings suggest that a combination of microarray analysis, qRT- PCR analysis, and QTL mapping can provide an efficient strategy to discover genes associated with Aspergillus flavus infection and aflatoxin accumulation.
Materials and Methods
Plant Materials and Experimental Designs
Maize inbred lines Mp313E, Mp04∶86, Va35, and B73 were maintained by the United States Department of Agriculture, Agricultural Research Service, Corn Host Plant Resistance Research Unit (USDA-ARS-CHPRRU) at Mississippi State University. Mp313E and Mp04∶86 are maize inbred lines showing resistance to Aspergillus flavus infection and aflatoxin accumulation. Va35 and B73 are elite maize germplasm but are susceptible to Aspergillus flavus. Mp04∶86 is a recombinant inbred line derived from a cross of maize lines Va35 and Mp715. Both Mp715 and Mp313E are used primarily as resources of resistance for maize breeding projects [31], [32]. For the microarray experiment, the field experimental design was a randomized complete block with split plot and three replications for each genotype. All maize lines were planted at the R. R. Foil Plant Science Farm at Mississippi State University. The four genotypes were planted in the main plots. The two treatments (inoculated and uninoculated) were applied to the corresponding subplots. All primary ears were self-pollinated. The inoculation was performed 14 days after self-pollination using the fungus Aspergillus flavus strain NRRL 3357 (ATCC # 200026; SRRC 167), a strain known to produce high levels of aflatoxin in corn grain [33]. The procedure of fungal culture preparation and the fungal inoculation with side-needle teqhnique was as described previously [30], [34]. Four days after inoculation, which was 18 days after self-pollination, kernels from inoculated and uninoculated primary ears were collected for RNA preparation. The experimental procedure for microarray hybridization and data acquisition followed Kelley et al [30]. All remaining primary ears from each plot were harvested at maturity and processed as previously described by Windham and Williams [35] for aflatoxin accumulation analysis.
The field experimental design for qRT-PCR analysis was a randomized complete block with three replicates for each genotype. The resistant Mp313E and the susceptible Va35 were used. Kernel samples were collected in the field over eight time points. The first collection was performed within three hours after the fungal inoculation (0 days after inoculation). The other collecting time points were at 1, 2, 3, 4, 7, 14, and 21 days after inoculation. Kernels were flash frozen in liquid nitrogen, ground into powder, and stored at –80°C for further analysis.
Maize Oligonucleotide Microarray Hybridization and Data Acquisition
The maize oligonucleotide arrays from the NSF Maize Oligonucleotide Array Project were used. Each set of the maize arrays contains two slides, MO-A-1-9 and MO-B-1-9, with 57,452 maize gene oligonucleotide probes altogether [36]. Each slide was hybridized with the inoculated and uninoculated samples of the same genotype from the same main plot. Three sets of slides for six samples were used for Mp313E and B73, respectively. Two sets of slides for four samples were used for Mp 04∶86 and Va35, respectively. An additional set of slide was used as a dye swap for each genotype. The experiment followed the procedure as described previously [30]. All recommendations of the minimum requirements for a microarray experiment (MIAME) checklist [37] were observed, and the microarray data have been deposited with the European Molecular Biology Laboratory (EMBL) – the European Bioinformatics Institute (EBI) (E-MTAB-766).
Microarray Data Analysis
All data were first analyzed with SAS Version 9.1.3 (SAS, Cary, NC) [18]. A more detailed description of the method can be found in Kelley et al [30]. Briefly, the median value for the intensity of each spot was log transformed and analyzed by analysis of variance appropriate as a split plot design. The main unit was genotype and the subunit was inoculation treatment. Dye was treated as a fixed effect in the model to account for differences in dyes. Estimates of expression ratios (I/U) for each gene of each genotype were obtained. F-test for Genotype x Inoculation Treatment interaction was used to address the null hypothesis and obtain the significance levels for the expressed genes. Here is an example for an equation of the null hypothesis between a resistant maize line (Mp313E) and a susceptible maize line (Va35). H0: Log (Mp313E-I) - Log (Mp313E-U) = Log (Va35-I) - Log (Va35-U), where I and U refer to inoculated and uninoculated, respectively. Finally, the log2 values of the expression ratios (I/U) for expressed genes in all genotypes were analyzed by principal component analysis (PCA) and plotted using R 2. 12. 1 [38].
The microarray data were also analyzed using the R package of the Linear Models for Microarray Data (LIMMA) [19]. For the preprocessing step, data were normalized by the within array print group loess method. Log ratio (M) and log intensity (A) were calculated. Highly significant genes (P<0.01) were found and ranked via the limFit and topTable functions.
Quantitative Real Time RT-PCR
To validate the expression levels of candidate genes obtained by microarray analysis, qRT-PCR [39] was conducted using the Roche LightCycler 480 instrument (Roche Applied Science). ThermoScript RT-PCR system (Invitrogen, #11146-024) was used to prepare cDNA samples. LightCycler 480 SYBR Green I Master kit (Roche Applied Science, #04 707 516 001) was used for the qRT-PCR reactions. The qRT-PCR program was as the following: 1) 1 cycle of 95°C for 5 min; 2) 45 cycles of 95°C for 10 sec, 60°C for 15 sec, 72°C for 15 sec; 3) 1 cycle of 95°C for 5 sec, 65°C for 1 min, 97°C at continuous; 4) 1 cycle of 40°C for 10 sec. The primer sequences for qRT-PCR are listed in Table2. Data were analyzed and plotted by using GenEx 5.0.1 [20]. The PCR efficiency correction and reference gene normalization followed the software instructions. Two housekeeping genes, Zea mays glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Zea mays RNA polymerase II largest subunit (RPII), were tested in this qRT-PCR study. The geNorm function in GenEx was used to determine which housekeeping gene had least variations among all the samples. The gene has the smallest M-value was used as a reference gene. For the qRT-PCR statistical analysis, the paired t-test (2-tail) was used to test the two means of the expression levels of each gene between Mp313E and Va35 samples. The null hypothesis is H0: no difference between the expression level means in Mp313E and in Va35 for the tested gene.
Maize Chromosome Bin Location Comparison of Candidate Genes and QTLs
Information of QTLs used in this study was obtained from two mapping populations studied previously. The Mp313E xVa35 population includes 216 F3 families and was evaluated for three years. A total of 15 QTL regions, located on chromosomes 1, 2, 3, 4, 6, and 9, have been identified (Figure7). Four QTLs on chromosome 1, 4 (2), and 9 were above a significance level of 23.58 in likelihood ratio [14, and Willcox et al. 2000, unpublished data]. Two regions (chromosome 1, bin 1.08–1.09) and chromosome 4 (bin 4.04–4.08) were identified in all three years. The chromosome 1 (bin 1.08–1.09) QTL was associated with the Va35 genotype of DNA marker. The chromosome 4 (bin 4.04–4.08) QTL was associated with the Mp313E genotype of DNA marker. The Mp313E x B73 population contains 210 F2∶3 families and was also evaluated for three years [15]. A total of seven QTL regions were identified. They are located on chromosome 1, 2, 3, 4, 5, and 6 (Figure 7). The two QTLs on chromosome 2 and 4 were most significant, followed by the two QTLs on chromosomes 3 and 5 in this population.
The genetic map of each maize chromosome is divided into 100 segments, namely bins, which are marked by the Core Bin Markers [40]. A bin comprises all loci between the two Core Bin Markers. The information of the mapped loci in each bin is available in the Maize Genetics and Genome database (the Maize GDB). We therefore placed QTLs from different populations on one chromosome bin map for display purpose (Figure 7). Different legends were used in Figure 7 to distinguish the population origins of the QTLs. The physical mapping locations of the Core Bin Markers are identified also in the Maize GDB. The chromosomal bin locations of the highly expressed genes were determined by searching for gene sequence positions in the Maize GDB and then comparing them with the physical positions of the Core Bin Markers used for QTL analysis. Figure 7 shows a combined maize chromosome bin map for the purpose of display and comparison of results from multiple experiments.
Acknowledgments
We thank Dr. Andy D. Perkins for the instructions on JWH’s LIMMA analysis work and Kristin Matzek for the lab assistance in qRT-PCR reactions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported through the USDA Agricultural Research Service (ARS) funded Specific Cooperative Agreement (No. 58-6406-6-0039) between USDA-ARS and Mississippi Agri & Forestry Exp Station (MAFES) at Mississippi State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wild CP, Hall AJ. Primary prevention of hepatocellular carcinoma in developing countries. Mutat Res. 2000;462:381–393. doi: 10.1016/s1383-5742(00)00027-2. [DOI] [PubMed] [Google Scholar]
- 2.Gourma H, Bullerman LB. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds. J Prot Ecol. 1995;58:1395–1404. doi: 10.4315/0362-028X-58.12.1395. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar D, Cary JW, Ehrlich K, Yu J, Cleveland TE. Understanding the genetics of regulation of aflatoxin production and Aspergillus flavus development. Mycopathologia. 2006;162:155–66. doi: 10.1007/s11046-006-0050-9. [DOI] [PubMed] [Google Scholar]
- 4.Cleveland TE, Yu J, Bhatnagar D, Chen ZY, Brown RL, et al. Progress in elucidating the molecular basis of the host plant–Aspergillus flavus interaction, a basis for devising strategies to reduce aflatoxin contamination in crops. J Toxicology. 2004;23:345–380. [Google Scholar]
- 5.Oliver RP, Ipcho SVS. Arabidopsis pathology breathes new life into the necrotrophs-vs.-biotrophs classification of fungal pathogens. Mol Plant Path. 2004;5:347–352. doi: 10.1111/j.1364-3703.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg JT. Programmed cell death in plant-pathogen interactions. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:525–545. doi: 10.1146/annurev.arplant.48.1.525. [DOI] [PubMed] [Google Scholar]
- 7.Piffanelli P, Devoto A, Schulze-Lefert P. Defence signaling pathways in cereals. Curr Opin Plant Biol. 1999;2:295–300. doi: 10.1016/S1369-5266(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 8.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 10.Lorang JM, Sweat TA, Wolpert TJ. Plant disease susceptibility conferred by a “resistance” gene. Proc Natl Acad Sci USA. 2007;104:14861–14866. doi: 10.1073/pnas.0702572104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faris JD, Zhang ZC, Lu HJ, Lu SW, Reddy L, et al. A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc Natl Acad Sci USA. 2010;107:13544–13549. doi: 10.1073/pnas.1004090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams WP. Breeding for resistance to aflatoxin accumulation in maize. Mycotoxin Res. 2006;22:27–32. doi: 10.1007/BF02954554. [DOI] [PubMed] [Google Scholar]
- 13.Williams WP, Windham GL. Registration of maize germplasm line Mp717. Crop Sci. 2006;46:1407–1408. [Google Scholar]
- 14.Davis GL, Windham GL, Williams WP. QTL for aflatoxin reduction in maize. Maize Genet Conf. 2000;41:22. [Google Scholar]
- 15.Brooks TD, Williams WP, Windham GL, Willcox MC, Abbas HK. Quantitative trait loci contributing resistance to aflatoxin accumulation in the maize inbred Mp313E. Crop Sci. 2005;45:171–174. [Google Scholar]
- 16.Warburton ML, Brooks TD, Krakowsky MD, ShanXY, Windham GL, et al. Identification and mapping of new sources of resistance to aflatoxin accumulation in maize. Crop Sci. 2009;49:1403–1408. [Google Scholar]
- 17.Schnable PS, Ware D, Fulton R, Stein J, Wei F, et al. The B73 maize genome: complexity, diversity and dynamics. Science. 2009;326:1112–1115. doi: 10.1126/science.1178534. [DOI] [PubMed] [Google Scholar]
- 18.Statistical Analysis Software, Copyright © 2011 SAS Institute Inc., SAS Campus Drive, Cary, North Carolina 27513, USA.
- 19.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology, Vol. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 20.0. 1. MultiD Analyses AB, Odinsgatan 28, SE-411 03 Göteborg, Sweden; GenEx Software 5. [Google Scholar]
- 21.Naqvi SMS, Park KS, Yi SY, Lee HW, Bok SH, et al. A glycine-rich RNA-binding protein gene is differentially expressed during acute hypersensitive response following Tobacco Mosaic Virus infection in tobacco. Plant Mol Biol. 1998;37:571–576. doi: 10.1023/a:1006031316476. [DOI] [PubMed] [Google Scholar]
- 22.Singh U, Deb D, Singh A, Grover A. Glycine-rich RNA binding protein of Oryza sativa inhibits growth of M15 E. coli cells. BMC research notes. 2011;4:18. doi: 10.1186/1756-0500-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freire MA, Pages M. Functional characteristics of the maize RNA-binding protein MA16. Plant Mol Biol. 1995;29:797–807. doi: 10.1007/BF00041169. [DOI] [PubMed] [Google Scholar]
- 24.Macias S, Bragulat M, Tardiff DL, Vilardell J. L30 binds the nascent RPL30 transcript to repress U2 snRNP recruitment. Mol Cell. 2008;30:732–742. doi: 10.1016/j.molcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Basha E, Jones C, Wysocki V, Vierling E. Mechanistic differences between two conserved classes of small heat shock proteins found in the plant cytosol. J Biol Chem. 2010;285:11489–11497. doi: 10.1074/jbc.M109.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia AV, Parker JE. Heaven’s gate: nuclear accessibility and activities of plant immune regulators. Trends in Plant Sci. 2009;14:479–487. doi: 10.1016/j.tplants.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Cheng YT, Germain H, Wiermer M, Bi DG, Xu F, et al. Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. The Plant Cell. 2009;21:2503–2516. doi: 10.1105/tpc.108.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lillehoj EB, Garcia WJ, Lambrow M. Aspergillus flavus infection and aflatoxin production in corn: Influence of trace elements. Applied Microbiol. 1974;28:763–767. doi: 10.1128/am.28.5.763-767.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pechanova O, Techan T, Williams WP, Luthe DS. Proteomic analysis of the maize rachis: Potential roles of constitutive and induced proteins in resistance to Aspergillus flavus and aflatoxin. Proteomics. 2011;11:114–127. doi: 10.1002/pmic.201000368. [DOI] [PubMed] [Google Scholar]
- 30.Kelley RY, Williams WP, Mylroie JE, Boykin DL, Hawkins LK, et al. Genomic Profile of Maize Response to Aspergillus flavus Infection. Toxin Rev. 2009;28:129–141. [Google Scholar]
- 31.Scott GE, Zummo N. Registration of Mp313E parental line of maize. Crop Sci. 1990;30:1378. [Google Scholar]
- 32.Williams WP, Windham GL. Registration of maize germplasm line Mp715. Crop Sci. 2001;41:1374–1375. [Google Scholar]
- 33.Windham GL, Williams WP. Evaluation of corn inbreds and advanced breeding lines for resistance to aflatoxin contamination in the field. Plant Dis. 2002;86:232–234. doi: 10.1094/PDIS.2002.86.3.232. [DOI] [PubMed] [Google Scholar]
- 34.Zummo N, Scott GE. Evaluation of field inoulation teqhniques for screening maize genotypes against kernel infection by Aspergillus flavus in Mississippi. Plant Dis. 1989;73:313–316. [Google Scholar]
- 35.Windham GL, Williams WP. Aspergillus flavus infection and accumulation in resistant and susceptible maize hybrids. Plant Dis. 1998;82:281–284. doi: 10.1094/PDIS.1998.82.3.281. [DOI] [PubMed] [Google Scholar]
- 36.Pontius JU, Wagner L, Schuler GD. The NCBI Handbook. Bethesda (MD): National Center for Biotechnology Information; 2003. UniGene: a unified view of the transcriptome. [Google Scholar]
- 37.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, et al. Minimum information about a microarray experiment (MIAME)–toward standards for microarray data. Nat Genet. 2001;29:365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 38.R Development Core Team. 2008. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardiner J, Coe EH, Melia-Hancock S, Hoisington DA, Chao S. Development of a core RFLP map in maize using an immortalized F2 population Genetics. 1993;134:917–930. doi: 10.1093/genetics/134.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]