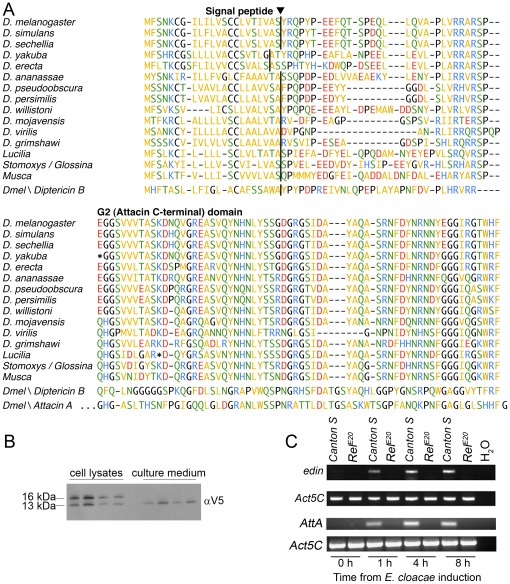
Figure 1. Edin is a Relish-dependently synthesized peptide, which is secreted from S2 cells.
(A–B) Edin contains a signal sequence and is secreted from S2 cells. (A) Edin sequences are aligned from 12 Drosophila species and three other dipterans. Diptericin B and Attacin A from D. melanogaster are also included in the alignment. The predicted signal peptidase cleavage sites [31] are marked. The sequences from the 12 Drosophila species are all from Clark et al. 2007 [32], except the D. mojavensis sequence which is derived from an EST sequence (EB600147). Modified gene models without introns were used for D. yakuba and D. willistoni. The Lucilia sericata sequence is derived from a single EST (FG360503). Three Stomoxys calcitrans ESTs (DN952426, DN952940, EZ048833) and one Glossina morsitans EST (AF368915) appear to contain overlapping sequence from the same gene. The Musca domestica sequence is an isoform represented by one EST (ES608713). (B) The signal sequence of Edin is cleaved before the peptide is secreted to the cell culture medium. S2 cells were transfected with a pMT-edin-V5 construct and the cell culture medium and cell lysates were analyzed with western blotting. Both full-length and cleaved forms were observed in the lysates while only the cleaved form was present in the medium. The V5 tag is located at the C-terminus of Edin. The blot represents 4 independent samples from which both cell lysates and culture medium were analyzed. (C) Edin is induced upon Enterobacter cloacae infection in Canton S flies but not in RelE20 flies. Canton S flies and RelE20-mutant flies were pricked with E. cloacae and total RNAs were extracted at the indicated time points. RT-PCR was performed and samples were electrophoresed on an agarose gel. Actin5C was used as a loading control and Attacin A as a positive control.