Abstract
Background
Finger-tapping has been widely studied using behavioral and neuroimaging paradigms. Evidence supports the use of finger-tapping as an endophenotype in schizophrenia, but its relationship with motor procedural learning remains unexplored. To our knowledge, this study presents the first use of index finger-tapping to study procedural learning in individuals with schizophrenia or schizoaffective disorder (SCZ/SZA) as compared to healthy controls.
Methods
A computerized index finger-tapping test was administered to 1169 SCZ/SZA patients (62% male, 88% right-handed), and 689 healthy controls (40% male, 93% right-handed). Number of taps per trial and learning slopes across trials for the dominant and non-dominant hands were examined for motor speed and procedural learning, respectively.
Results
Both healthy controls and SCZ/SZA patients demonstrated procedural learning for their dominant hand but not for their non-dominant hand. In addition, patients showed a greater capacity for procedural learning even though they demonstrated more variability in procedural learning compared to healthy controls. Left-handers of both groups performed better than right-handers and had less variability in mean number of taps between non-dominant and dominant hands. Males also had less variability in mean tap count between dominant and non-dominant hands than females. As expected, patients had a lower mean number of taps than healthy controls, males outperformed females and dominant-hand trials had more mean taps than non-dominant hand trials in both groups.
Conclusions
The index finger-tapping test can measure both motor speed and procedural learning, and motor procedural learning may be intact in SCZ/SZA patients.
Keywords: Schizophrenia, Procedural learning, Finger-tapping, Motor function, Sensorimotor function, Handedness
1. Introduction
Finger-tapping has been studied in healthy individuals using behavioral (Ashendorf et al., 2009; Jimenez-Jimenez et al., 2011; Peters and Durding, 1979) and neuroimaging (L. Jancke et al., 2006; Witt et al., 2008) paradigms as a measure of sensorimotor brain function, recruiting the primary motor cortex, cerebellum, pre-supplementary motor area and premotor cortex (Boecker et al., 1994; Deiber et al., 1999; J. Jancke et al., 1998; L. Jancke et al., 2006; Moritz et al., 2000; Rao et al., 1996; Sadato et al., 1997; Witt et al., 2008). In addition, tapping ability has been correlated with hand preferences, with better performance (i.e. more taps or less intertap variations) for the dominant hand (Carlier et al., 1993; Nalçaci et al., 2001; Peters, 1980). Furthermore, right-handers have greater differences between hands in tapping rate and intertap variability than non right-handers (Nalçaci et al., 2001; Peters and Durding, 1979; Schmidt et al., 2000). In adults, males outperform females (R.C. Gur et al., 2010; Saykin et al., 1995; Shimoyama et al., 1990). There is a decline in tapping ability with age, alongside other motor functions, possibly due to natural dopaminergic D2 activity decline in the caudate and putamen (Aoki and Fukuoka, 2010; Volkow et al., 1998).
Finger-tapping tests have been used to evaluate sensorimotor function in patients with schizophrenia, who show an overall deficit as measured by number of taps or intertap intervals (Calkins et al., 2010; Flyckt et al., 1999; Greenwood et al., 2007). This may be related to impairments in motor temporal processing (Carroll et al., 2009), dysfunctional sensorimotor cortex activation (Schroder et al., 1999) and abnormalities in motor neural pathways (Flyckt et al., 2000). Finger-tapping performance has been considered as a candidate endophenotype because unaffected first-degree relatives perform at an intermediate level, scoring higher than schizophrenia patients but lower than healthy controls (Calkins et al., 2007; Calkins et al., 2010; Flyckt et al., 1999; Flyckt et al., 2000; Greenwood et al., 2007; R.E. Gur, Calkins, et al., 2007). Nevertheless, the relationship between these sensorimotor deficits in schizophrenia and motor procedural learning remains unexplored.
1.1 Procedural learning and finger-tapping performance in schizophrenia
In addition to evaluating motor speed, finger-tapping performance can be scrutinized over time to evaluate procedural learning, i.e. implicitly learning a motor or cognitive procedure by repetition, up to the point of automation (Squire, 1986). In the finger-tapping test, procedural learning may account for improved tapping performance across test trials due to tapping repetition without the subject’s declarative knowledge of the learning involved.
Few studies have used motor sequence tests (MST) to investigate procedural learning in schizophrenia and healthy controls. These studies assessed the left-hand only, using finger-tapping multiple-digit sequences with re-assessments between 5 minutes and 24 hours intervals, unlike more common non-sequence based, single-digit finger-tapping tests (Hotermans et al., 2008; Hotermans et al., 2006; Manoach et al., 2004; Manoach et al., 2010). Other tests applied to assess procedural learning in schizophrenia include the Tower of Toronto (Bedard et al., 2000; Purdon et al., 2003), Tower of Hanoï (Goldberg et al., 1990), mirror drawing tasks (Bedard et al., 1996a; Bedard et al., 2000; Paquet et al., 2004; Scherer et al., 2004), serial reaction time tasks (Harris et al., 2009; Kern et al., 1998; Siegert et al., 2008), periodic sequence learning tasks (Kumari et al., 2002), rotary pursuit (Kern et al., 1998; B.L. Schwartz et al., 1996), computed visual tracking tasks (Paquet et al., 2004) and non-motor (verbal) procedural learning tests (Remillard et al., 2010). The results from these studies suggest that patients with schizophrenia demonstrate procedural learning, but that their learning profile may be impaired compared to healthy controls, potentially due to medication (Bedard et al., 2000; Kumari et al., 2002; Purdon et al., 2003; Purdon et al., 2002; Remillard et al., 2010; Stip, 2006).
Medication effects on procedural learning have been attributed mainly to the antagonist activity of high affinity D2 receptor antipsychotics on dopaminergic dorsal striatum pathways (Bedard et al., 1996a; Bedard et al., 2000; Harris et al., 2009; Paquet et al., 2004; Purdon et al., 2003; Purdon et al., 2002; Remillard et al., 2010; Zedkova et al., 2006). This has also been supported by a double-blind study with healthy participants who showed improvement in procedural learning following administration of a dopamine agonist and no significant procedural learning for the group given a dopamine antagonist (Kumari et al., 1997). However, procedural learning deficits have been reported in drug-naïve patients (Scherer et al., 2003) or during acute phases of psychosis but not during remission (Exner, Boucsein, et al., 2006; Exner, Weniger, et al., 2006). Notably, medication related effects have not prevented subjects from eventually succeeding on procedural learning tasks or performing similarly to control groups (Bedard et al., 1996b; Bedard et al., 2000; Goldberg et al., 1990; Green et al., 1997; Harris et al., 2009; Kumari et al., 1997; Scherer et al., 2004; M. Schwartz and Regan, 1996).
In summary, while motor-speed deficits in finger-tapping have been reported in schizophrenia, finger-tapping performance has not been used to examine procedural learning. This study capitalizes on the availability of data from large well-characterized samples collected by three genetic consortia that applied the Penn Computerized Neurocognitive Battery (CNB) (R.C. Gur et al., 2001; R.C. Gur et al., 2010). The results for the CNB are published (Aliyu et al., 2006; Almasy et al., 2008; Calkins et al., 2007; Calkins et al., 2010; R.E. Gur, Calkins, et al., 2007; R.E. Gur, Nirngaonkar, et al., 2007). Here, we examine one of the tests, the finger-tapping test of motor speed, to investigate procedural learning in individuals with schizophrenia. Our goals were: a) to compare motor speed, operationalized as number of key taps in 10 second intervals, between healthy controls and patients for dominant and non-dominant hands; b) to investigate whether procedural learning, defined as the rate of improvement (learning slope) over trials, is evident in the finger-tapping test; and c) to compare the procedural learning profiles of patients with that of healthy controls.
We hypothesized that healthy controls would have more taps than patients with schizophrenia, males would outperform females, and the dominant hand would outperform the non-dominant hand in both groups. We further hypothesized that patients and healthy controls would demonstrate procedural learning, and explored whether their learning slopes differed.
2. Methods
2.1 Participants
The sample of 1169 patients with schizophrenia or schizoaffective disorder (SCZ/SZA) and 689 healthy controls (HC) is from the consortia of Multiplex Multigenerational Investigation (MGI) (Almasy et al., 2008; R.E. Gur, Nirngaonkar, et al., 2007), Project Among Africa-Americans to Explore Risks for Schizophrenia (PAARTNERS) (Aliyu et al., 2007; Calkins et al., 2010), and the Consortium on the Genetics of Schizophrenia (COGS) (Calkins et al., 2007; R.E. Gur, Calkins, et al., 2007). Project specific inclusion/exclusion criteria and assessment methods are detailed in previously published articles (Aliyu et al., 2007; Calkins et al., 2007; Calkins et al., 2010; R.E. Gur, Calkins, et al., 2007; R.E. Gur, Nimgaonkar, et al., 2007). All research centers received Institutional Review Board (IRB) approval.
Participants’ characteristics are shown in Table 1. Out of the SCZ/SZA group, 10% of the male participants had schizoaffective disorder (63 right-handers and 10 left-handers); and 24% of the female participants had schizoaffective disorder (96 right-handers and 10 left-handers). SCZ/SZA and HC participants were matched in age but significantly differed with respect to sex distribution, race, handedness, level of education and parental education (p<0.001).
Table 1.
Participants’ demographic information:
| HC participants (N=689) |
SCZ/SZA participants (N=1169) |
p value | ||
|---|---|---|---|---|
| Sex | Male (% total) | 276 (40.1%) | 729 (62.4%) | < 0.0001a |
| Female (% total) | 413 (59.9%) | 440 (37.6%) | ||
| Race | White (% total) | 234 (34.1%) | 206 (17.7%) | < 0.0001a |
| African American (% total) |
368 (53.6%) | 902 (77.6%) | ||
| American Indian / Alaskan native (% total) |
4 (0.6%) | 1 (0.1%) | ||
| Asian (% total) | 29 (4.2%) | 13 (1.1%) | ||
| Mixed race (more than one) (% total) |
52 (7.6%) | 41 (3.5%) | ||
| Handedness | Right handed (% total) |
643 (93.3%) | 1028 (87.9%) | 0.0002a |
| Left handed (% total) |
46 (6.7%) | 141 (12.1%) | ||
| Age (years) | Overall Mean (SD) | 38.6 (14.3) | 38.5 (11.8) | 0.8902b |
| Male Mean (SD) | 37.6 (13.5) | 36.6 (11.8) | ||
| Female Mean (SD) | 39.2 (14.9) | 41.7 (11.2) | ||
| Education level (years) | Mean (SD) | 14.3 (2.8) | 12.0 (2.4) | < 0.0001b |
| Mothers Education (years) | Mean (SD) | 13.0 (3.5) | 11.7 (3.6) | < 0.0001b |
| Fathers Education (years) | Mean (SD) | 13.1 (3.8) | 11.4 (4.2) | < 0.0001b |
! 2 test.
T-test.
Abbreviations: HC, healthy control participants; SCZ/SZA, schizophrenia or schizoaffective disorder patients.
Handedness was assessed through self-report at the time of testing. Participants were asked whether they were right-handed, left-handed or whether they used both hands for writing, as preferred writing hand yields a reliable measure of handedness based on the Lateral Dominance Examination (LDE) (Dodrill and Thoreson, 1993). Eighteen SCZ/SZA and eight HC participants who were ambidextrous were excluded from analyses. Most patients (99%) were treated with antipsychotics, 60% with second generation and 40% with first generation agents.
2.2 Test design: Computerized finger-tapping test (CTAP)
Participants took the Computerized Finger-tapping Test (CTAP) (Coleman et al., 1997; R.C. Gur et al., 2010) using the PowerLaboratory® platform (Chute DL and Westall RF, 1997) in a Macintosh® computer as part of the Penn Computerized Neurocognitive Battery (CNB) following standard administration procedures (Gur et al., 2010)a. A test administrator reads instructions on the screen to the participant, asking him/her to press the spacebar as quickly as s/he can using only the index finger of the dominant or non-dominant hand, with a hand position that targets movement of the index finger only. The participant practices one trial with each hand while the test administrator verifies his/her hand position. During the test, the first trial is done with the dominant hand and then alternates with the non-dominant hand in a series of 10 trials (5 trials per hand). Each trial lasts 10 seconds. The number of taps per trial is recorded and uploaded to a data repository. Only tests where hand position was correct and the participant showed good effort are included in our analysis. The CTAP test, including practice instructions, lasts approximately 7 minutes. The test trials themselves last approximately 4 minutes.
2.3 Statistical analysis
Statistical analysis applied SAS® software, Version 9.2 of the SAS System for Linux (SAS Institute Inc., Cary, NC, USA).
2.3.1. Finger-tapping performance analysis
Trials with less than 10 taps were excluded from analysis since they are likely to represent technical complications. Over 99.7% of trials met this inclusion criteria: for controls, 6881 out of 6890 trials; and 11654 out of 11690 trials for patients.
Finger-tapping performance analysis used SAS® PROC GLM (general linear model). A repeated measures analysis of variance (ANOVA) examined between-group (diagnosis, sex, age, handedness) and within-subjects (dominant vs. non-dominant hand) effects, as well as interactions of the between-group and within-subject effects.
2.3.2. Procedural learning analysis
Procedural learning was defined as the rate of change (learning slope) across trials of dominant and non-dominant hands. The data was structured as 10 records per participant with one record per trial and number of taps as the outcome. The distribution of the number of taps was approximately normal, thus SAS® PROC MIXED was used to fit random coefficient models (SAS Institute Inc, 2010; Wolfinger and Ming, 1995). While adjusting for age group, we tested the interactions between trial and diagnosis, sex, handedness and hand dominance (dominant vs. non-dominant hand).
To assess variability within procedural learning, the root mean squared error (rMSE) of the participant-specific regression line was calculated for both dominant and non-dominant hands. The rMSE was compared between HC and SCZ/SZA participants using the Wilcoxon rank sum test, stratified by hand dominance, since the distribution of rMSE was right-skewed.
3. Results
3.1. Finger-tapping performance scores
3.1.1 Between-group comparison
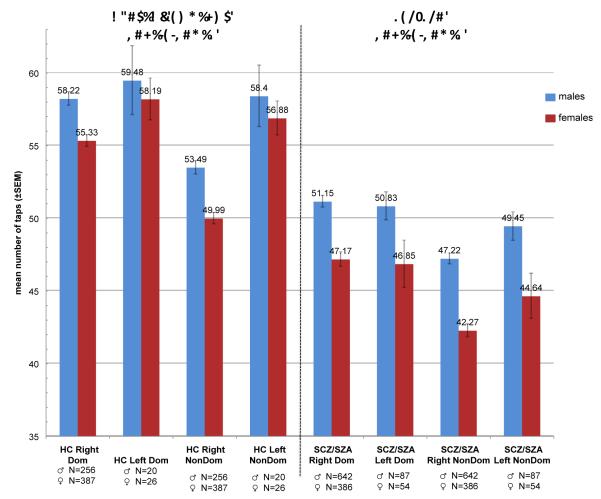
The distribution of the mean number of taps for both dominant and non-dominant hands was approximately normal, legitimizing ANOVA. Age group (younger than 25 years old, 25-34, 35-44, 45-54 and 55 or older) was entered as a covariate due to differences in motor speed across age (Aoki and Fukuoka, 2010; R.C. Gur et al., 2010; Volkow et al., 1998). The results (Table 2) showed a robust effect of diagnosis, p<0.0001, patients slower than controls; sex, p<0.0001, females slower than males; and handedness, p=0.0284, right-handers slower than left-handers. The covariate of age group was also highly significant, p<0.0001, with slowing beginning in the 35-44 age group. No interaction of diagnosis, sex and handedness was significant.
Table 2.
Repeated Measures Analysis of Variance and Effect Sizes
| Tests of Hypotheses for Between Subjects Effects | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect | DF | Type III SS | Mean Square | F Value | Pr > F | Generalized Eta-squared |
| Diagnosis | 1 | 43208.04 | 43208.04 | 293.61 | <.0001 | 0.11883 |
| Sex | 1 | 9766.47 | 9766.47 | 66.37 | <.0001 | 0.02686 |
| Handedness | 1 | 707.85 | 707.85 | 4.81 | 0.0284 | 0.00195 |
| Age | 4 | 15761.05 | 3940.26 | 26.78 | <.0001 | 0.04335 |
| Error | 1850 | 272249.38 | 147.16 | |||
Figure 1 illustrates the mean number of taps for CTAP trials and standard error of the means (SEM) based on diagnosis, sex and handedness. Sample size for males and females is recorded on the bottom of the x-axis according to each group of participants:
Figure 1.
Abbreviations: ♂ , males; ♀ , females; HC, healthy controls; SCZ/SZA, schizophrenia or schizoaffective disorder patients; Right Dom, dominant hand trials for right-handed participants; Left Dom, dominant hand trials for left-handed participants; Right NonDom, non-dominant hand trials for right-handed participants; Left NonDom, non-dominant hand trials for left-handed participants
3.1.2 Within-subjects analysis for dominant and non-dominant hands
Table 3 shows the analysis of within-subjects effects, where number of taps for dominant versus non-dominant hand trials (DomHand) was compared. The results yielded a pronounced main effect of hand dominance, with more taps produced by the dominant hand, as well as strong interaction effects of hand-dominance × handedness (DomHand × Hand), right-handers showed greater difference in mean number of taps between dominant and non-dominant hand trials compared to left-handers; and hand-dominance × sex (DomHand × Sex), females showed greater differences between dominant and non-dominant hand trials compared to males.
Table 3.
Repeated Measures Analysis of Variance - Univariate Tests and effect sizes
| Univariate Tests of Hypotheses for Within Subject Effects | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Effect | DF | Type III SS |
Mean Square |
F Value |
Pr > F | Generalized Eta-squared |
| DomHand | 1 | 3240.94 | 3240.94 | 286.16 | <.0001 | 0.43794 |
| DomHand × Diagnosis | 1 | 41.3 | 41.3 | 3.65 | 0.0563 | 0.00011 |
| DomHand × Sex | 1 | 158.88 | 158.88 | 14.03 | 0.0002 | 0.00044 |
| DomHand × Handedness | 1 | 720.9 | 720.9 | 63.65 | <.0001 | 0.00198 |
| DomHand × Age | 4 | 34.61 | 8.65 | 0.76 | 0.5486 | 0.0001 |
| Error(DomHand) | 1850 | 20952.73 | 11.33 | |||
Abreviations:
DomHand: dominant vs nondominant hand the dominant hand, as well as strong interaction effects of hand-dominance × handedness (DomHand × Hand), right-handers showed greater difference in mean number of taps between dominant and non-dominant hand trials compared to lefthanders; and hand-dominance × sex (DomHand × Sex), females showed greater differences between dominant and non-dominant hand trials compared to males.
3.2. Procedural learning analysis
3.2.1 Learning slopes
Tables 4 and 5 present the random coefficient models examining the effects of diagnosis, sex, handedness (Hand), dominant vs. non-dominant hand (Hand Dominance), age, and trial on the number of taps per trial as well as their interactions, except for interactions with age, because healthy controls and SCZ/SZA participants were age-matched. There were main effects for diagnosis (F(1,15000)=129.31, p<0.0001), sex (F(1,15000)=64.45, p<0.0001), and age (F(4,15000)=30.37, p<0.0001). There was a strong effect of trial × dominant hand (F(1, 15000)=32.53, p<0.0001), healthy controls and patients demonstrated procedural learning with their dominant hand but not with their non-dominant hand (Table 5); and there was an effect of trial × diagnosis (F(1, 15000)=4.73, p=0.0296), patients showed significantly more procedural learning than healthy controls. We tested the 3-way interaction between trial × diagnosis × dominant hand, but this was not statistically significant (p=0.54), and therefore dropped from the final model.
Table 4.
PROC MIXED solution for fixed effects
| Factor | Beta Estimate (SE) |
P-value |
|---|---|---|
| Intercept | 51.69 (1.42) | <0.0001 |
| Diagnosis (SCZ/SZA vs. HC) | −10.67 (1.49) | <0.0001 |
| Sex (Male vs. Female) | 3.77 (0.42) | <0.0001 |
| DomHand | −5.50 (1.34) | <0.0001 |
| Handedness | 1.09 (0.39) | 0.0058 |
| Age Group (< 25 vs. 55+) | 5.81 (0.77) | <0.0001 |
| Age Group (25-34 vs. 55+) | 6.08 (0.73) | <0.0001 |
| Age Group (35-44 vs. 55+) | 4.51 (0.73) | <0.0001 |
| Age Group (45-54 vs. 55+) | 1.45 (0.74) | 0.048 |
| Diagnosis × DomHand | 3.60 (1.55) | 0.0203 |
| Diagnosis × Handedness | 0.64 (0.44) | 0.1468 |
| Sex × Handedness | −0.83 (0.12) | <0.0001 |
| Handedness × DomHand | 3.86 (0.39) | <0.0001 |
| Diagnosis × Handedness × | −1.25 (0.46) | 0.0062 |
| DomHand | ||
| Trial (slope) | −0.11 (0.05) | 0.044 |
| Trial × Diagnosis | 0.13 (0.06) | 0.0296 |
| Trial × DomHand | 0.24 (0.04) | <0.0001 |
Abbreviations
HC: healthy control
participants;
SCZ/SZA: schizophrenia or schizoaffective disorder patients. DomHand: dominant vs nondominant hand
Table 5.
Slope estimates for linear contrasts:
| Beta Estimate (SE) | P-value | |
|---|---|---|
| slope for SCZ/SZA Dom | 0.27 (0.04) | <0.0001 |
| slope for SCZ/SZA NonDom | 0.03 (0.04) | 0.5419 |
| slope for HC Dom | 0.14 (0.05) | 0.0096 |
| slope for HC NonDom | −0.11 (0.05) | 0.044 |
| slope SCZ/SZA Dom vs. HC Dom | 0.13 (0.06) | 0.0296 |
| slope SCZ/SZA NonDom vs. HC NonDom | 0.13 (0.06) | 0.0296 |
Abbreviations
HC: healthy control participants;
SCZ/SZA: schizophrenia or
schizoaffective disorder patients;
3.2.2 Error variability across tapping trials
To further investigate procedural learning based on diagnosis, we calculated the rMSE of the participant-specific regression lines for both dominant and non-dominant hands. A larger rMSE meant more error about the regression line and hence more variability in procedural learning. Healthy controls had significantly less variability across their learning slopes compared to patients for both the dominant hand (Wilcoxon rank sum Z=-8.1, p<0.0001) and non-dominant hand (Wilcoxon rank sum Z=-7.3, p<0.0001), see Table 6.
Table 6.
Root Mean Squared Errors for Dominant Hand and Non-Dominant Hand, stratified by diagnosis:
| Diagnosis | N | Variable | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| HC | 689 | rMSE: Dominant | 2.11 | 1.85 | 1.7 | 0.32 | 24.28 |
| rMSE: Non- Dominant |
2.05 | 1.71 | 1.62 | 0 | 20.01 | ||
| SCZ/SZA | 1169 | rMSE: Dominant | 2.89 | 2.34 | 2.22 | 0.32 | 18.05 |
| rMSE: Non- Dominant |
2.66 | 2.02 | 2.18 | 0 | 25.62 |
Abbreviations: HC, healthy control participants; SCZ/SZA, schizophrenia or schizoaffective disorder patients
4. Discussion
The current study assessed procedural learning and motor speed with a computerized index-finger tapping test (CTAP) (R.C. Gur et al., 2010). Expectedly, participants with schizophrenia or schizoaffective disorder (SCZ/SZA) showed slower tapping speed (i.e. less taps) than healthy controls (HC). However, both groups demonstrated procedural learning with their dominant hand but not with their non-dominant hand. Notably, SCZ/SZA participants showed significantly more procedural learning than healthy controls, although they also had more variability on their procedural learning profiles. Our results indicate that procedural learning is intact in schizophrenia, and thus are encouraging in establishing a building block on which to construct rehabilitation efforts for patients.
Supporting the validity of the novel observation, the overall CTAP performance results were consistent with available data on finger-tapping tests. The main effects of diagnosis favoring controls over SCZ/SZA participants and of sex, favoring males over females, are robust findings in the literature (Calkins et al., 2010; Flyckt et al., 1999; Greenwood et al., 2007; R.C. Gur et al., 2010; Saykin et al., 1995; Shimoyama et al., 1990). The effect of handedness, demonstrating a smaller difference in speed performances between dominant and non-dominant hands for left-handers compared to right-handers has been reported in fewer studies (Nalçaci et al., 2001; Peters, 1980; Peters and Durding, 1979; Schmidt et al., 2000). The effect of sex on hand dominance variability, showing that males have less variability between dominant and non-dominant hands than females, is a novel finding to our knowledge. However, males have been reported to tap more regularly overall than females (Schmidt et al., 2000). Notably, these effects did not interact with diagnosis.
Procedural learning has been reported on finger-tapping motor sequence tests in schizophrenia and healthy controls within 5 to 30 minutes after a single training session using the non-dominant hand (Hotermans et al., 2006; Manoach et al., 2010). However, our finger-tapping test trials last approximately 4 minutes, thus suggesting that procedural learning is evident earlier. Remarkably, patients showed significantly more procedural learning than controls, even though they had a lower mean number of taps. They also showed a greater variability in procedural learning, which would be disruptive to this apparently greater potential to improve. Possibly, these are regression to the mean effects potentially related to the fact that patients had a lower initial mean number of taps, and hence, a greater opportunity to improve, while ceiling effects would limit how many taps are possible in 10 seconds in both groups, irrespective of practice (Aoki et al., 2005). Notably, however, the diagnosis x trial x hand interaction was not significant, which militates against regression to the mean effect as a major determinant.
Although both groups showed procedural learning with the dominant hand, it is unclear why, with the non-dominant hand, healthy controls showed a decline in procedural learning (negative slope) and patients showed a slope not significantly different from zero. Peters (1980) argued that time spent in the reversal portion of tapping (i.e. controlling the muscles used to prepare the finger for the next tap) is responsible for hand differences in tapping, and not fatigue or external sensory factors. Furthermore, Koeneke et al. (2009) proposed two levels of motor processing: “lower effector-related” level and “higher task-related” level. The former relates to the neuro-muscular pathways involved in control of the finger as it repeats tapping, the latter refers to a motor pre-programming of muscles which may be transferrable between hands and removed from the former (Koeneke et al., 2009). Therefore, the lack of procedural learning we observed on the non-dominant hand may be due to less efficient “lower effect-related level” control of flexor and extensor tapping muscles. Notably, we did not observe the “higher task-related level” intermanual transfer effects predicted by Koeneke et al. (2009). However, this may be due to limitations in our paradigm, as minimal training was offered prior to testing compared to the two weeks training in Koeneke et al. (2009).
Our study has several limitations. There is evidence for medication effects on procedural learning, especially high affinity D2 receptor antipsychotics (Kumari et al., 1997; Purdon et al., 2003). In our large scale study not designed specifically to examine medication effects, medication was not controlled and we are unable to establish its effects on procedural learning. We also limited our examination to patients and controls and have not evaluated family members. A functional neuroimaging study examined procedural learning in unaffected siblings of schizophrenia participants, and found that they showed reduced activity in prefrontal cortical regions similarly to schizophrenia patients. Notably, both groups performed similarly to healthy controls (Woodward et al., 2007; Zedkova et al., 2006). Another study has demonstrated differences between unmedicated first episode psychosis patients and controls in brain activation to procedural learning in the frontal cortex (Purdon et al., 2011). Therefore, since there is support for the use of finger tapping as a neurocognitive endophenotype for schizophrenia (Calkins et al., 2007; Calkins et al., 2010; Flyckt et al., 1999; Flyckt et al., 2000; Greenwood et al., 2007; R.E. Gur, Calkins, et al., 2007), future studies could consider an analysis of unaffected siblings to determine whether CTAP procedural learning performance or brain activation profiles are heritable. It is also important to note that generalizations of our findings may need to consider the ancestral composition of our sample, which encompasses a range of ethnic backgrounds, the majority of African American descent (77.6% of patients and 53.6% of controls), followed by people of Caucasian descent (17.7% of patients and 34.1% of controls), mixed ethnicity, Asian and American Indian or Alaskan native.
This study supports the use of finger-tapping as a test of motor speed and procedural learning in healthy controls and patients with schizophrenia or schizoaffective disorder. Furthermore, it supports the notion that SCZ/SZA patients can learn motor skills that involve procedural learning, even in a task they perform more poorly than healthy controls. This is an encouraging finding in light of the broad spectrum of deficits seen in schizophrenia. Future research may consider the effects of antipsychotic medication on procedural learning and examine the heritability of procedural learning as reflected in finger-tapping and more complex tests.
Acknowledgements
The authors thank the participants, research personnel and staff from PAARTNERS, COGS and MGI. This collective effort has made the study possible and we are greatly thankful to each of these individuals.
Role of funding source
The CTAP is included in the following grants supported by the National Institute of Mental Health (NIMH).
PAARTNERS project: RO1s MH66006 (L. Dianne Bradford), MH66278 (Bernie Devlin), MH066049 (Neil Edwards), MH66181-03 (Rodney Go), MH66121 (Raquel Gur), MH066005 (Joseph Kwentus), MH66050 (Joseph McEvoy), MH66263 (Vishwajit Nimgaonkar) and MH66004 (Alberto Santos). COGS project: RO1s MH065562 and MH43518 (Larry Seidman), MH065554 (Larry Siever), MH65707 (Michael Green), MH065571 (David Braff), MH65588 (Ann Olincy), MH65578 (Raquel Gur) and MH65558 (Debby Tsuang). MGI project: MH49142 (Raquel Gur), MH063480 (Vishwajit Nimgaonkar) and MH61622 (Laura Almasy). Farzin Irani (T32MH019112) was supported by NIMH training grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The CTAP is available to the scientific community for research purposes with IRB compliance (or equivalent ethics approval), along other computerized neurocognitive tests, through the PennCNP® webpage https://penncnp.med.upenn.edu/request.pl (R.C. Gur et al., 2001; R.C. Gur et al., 2010)
Conflict of interest
None of the authors have any actual or potential conflicts of interest to disclose.
Contributors
All authors significantly contributed to, reviewed and approved this manuscript. Its final version is credited to their collective efforts.
FNDS took part in conceptualization and data analysis, and wrote the first and final manuscript drafts. FI took part in conceptualization and data analysis, and helped write the manuscript. JR performed data processing and edited the manuscript. CMB carried statistical analysis and helped write the manuscript. WCB supervised the statistical analysis and did manuscript editing. REG took part in conceptualization and data analysis, integrated information across collaborative sites and helped write and edit the manuscript. RCG is the senior contributor for study conceptualization, implementation, data analysis and manuscript writing and editing.
References
- Aliyu MH, Calkins ME, Swanson CL, Lyons PD, Savage RM, May R, Wiener H, Devlin B, Nimgaonkar VL, Ragland JD, Gur RE, Gur RC, Bradford LD, Edwards N, Kwentus J, McEvoy JP, Santos AB, McCleod-Bryant S, Tennison C, Go RCP, Grp PS. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): Recruitment and assessment methods. Schizophr. Res. 2006;87(1-3):32–44. doi: 10.1016/j.schres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Aliyu MH, Calkins ME, Swanson CL, Lyons PD, Savage RM, May R, Wiener H, Devlin B, Nimgaonkar VL, Ragland JD, Gur RE, Gur RC, Bradford LD, Edwards N, Kwentus J, McEvoy JP, Santos AB, McCleod-Bryant S, Tennison C, Go RP. Project among African-Americans to explore risks for schizophrenia (PAARTNERS): Recruitment and assessment methods. Schizophr. Res. 2007;90(1-3):369–369. doi: 10.1016/j.schres.2006.06.027. vol 87, pg 32, 2006. [DOI] [PubMed] [Google Scholar]
- Almasy L, Gur RC, Haack K, Cole SA, Calkins ME, Peralta JM, Hare E, Prasad K, Pogue-Geile MF, Nimgaonkar V, Gur RE. A genome screen for quantitative trait loci influencing schizophrenia and neurocognitive phenotypes. A. J. Psychiatry. 2008;165(9):1185–1192. doi: 10.1176/appi.ajp.2008.07121869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Fukuoka Y. Finger Tapping Ability in Healthy Elderly and Young Adults. Med. Sci. Sports Exerc. 2010;42(3):449–455. doi: 10.1249/MSS.0b013e3181b7f3e1. [DOI] [PubMed] [Google Scholar]
- Aoki T, Furuya S, Kinoshita H. Finger-tapping ability in male and female pianists and nonmusician controls. Motor Control. 2005;9(1):23–39. doi: 10.1123/mcj.9.1.23. [DOI] [PubMed] [Google Scholar]
- Ashendorf L, Vanderslice-Barr JL, McCaffrey RJ. Motor tests and cognition in healthy older adults. Appl Neuropsychol. 2009;16(3):171–176. doi: 10.1080/09084280903098562. [DOI] [PubMed] [Google Scholar]
- Bedard MA, Scherer H, Delorimier J, Stip E, Lalonde P. Differential effects of D2- and D4-blocking neuroleptics on the procedural learning of schizophrenic patients. Can J Psychiatry. 1996a;41(7 Suppl 1):S21–24. doi: 10.1177/070674379604100704. [DOI] [PubMed] [Google Scholar]
- Bedard MA, Scherer H, Delorimier J, Stip E, Lalonde P. Differential effects of D-2- and D-4-blocking neuroleptics on the procedural learning of schizophrenic patients. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 1996b;41(7):S21–S24. doi: 10.1177/070674379604100704. [DOI] [PubMed] [Google Scholar]
- Bedard MA, Scherer H, Stip E, Cohen H, Rodriguez JP, Richer F. Procedural learning in schizophrenia: Further consideration on the deleterious effect of neuroleptics. Brain Cogn. 2000;43(1-3):31–39. [PubMed] [Google Scholar]
- Boecker H, Kleinschmidt A, Requardt M, Hanicke W, Merboldt KD, Frahm J. Functional Cooperativity of Human Cortical Motor Areas during Self-Paced Simple Finger Movements - a High-Resolution Mri Study. Brain. 1994;117:1231–1239. doi: 10.1093/brain/117.6.1231. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. The consortium on the genetics of endophenotypes in schizophrenia: Model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr. Bull. 2007;33(1):33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins ME, Tepper P, Gur RC, Ragland JD, Klei L, Wiener HW, Richard J, Savage RM, Allen TB, O’Jile J, Devlin B, Kwentus J, Aliyu MH, Bradford LD, Edwards N, Lyons PD, Nimgaonkar VL, Santos AB, Go RCP, Gur RE. Project Among African-Americans to Explore Risks for Schizophrenia (PAARTNERS): Evidence for Impairment and Heritability of Neurocognitive Functioning in Families of Schizophrenia Patients. A. J. Psychiatry. 2010;167(4):459–472. doi: 10.1176/appi.ajp.2009.08091351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M, Dumont AM, Beau J, Michel F. Hand Performance of French Children on a Finger-Tapping Test in Relation to Handedness, Sex, and Age. Percept. Mot. Skills. 1993;76(3):931–940. doi: 10.2466/pms.1993.76.3.931. [DOI] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71(3):345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chute DL, Westall RF. Power Laboratory. MacLaboratory Incorporated; Devon PA: 1997. [Google Scholar]
- Coleman AR, Moberg PJ, Ragland JD, Gur RC. Comparison of the Halstead-Reitan and Infrared Light Beam Finger Tappers. Assessment. 1997;4(3):277–286. doi: 10.1177/107319119700400307. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J. Neurophysiol. 1999;81(6):3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Thoreson NS. Reliability of the Lateral Dominance Examination. J. Clin. Exp. Neuropsychol. 1993;15(2):183–190. doi: 10.1080/01688639308402556. [DOI] [PubMed] [Google Scholar]
- Exner C, Boucsein K, Degner D, Irle E. State-dependent implicit learning deficit in schizophrenia: evidence from 20-month follow-up. Psychiatry Res. 2006;142(1):39–52. doi: 10.1016/j.psychres.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr. Res. 2006;84(2-3):386–396. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Flyckt L, Sydow O, Bjerkenstedt L, Edman G, Rydin E, Wiesel FA. Neurological signs and psychomotor performance in patients with schizophrenia, their relatives and healthy controls. Psychiatry Res. 1999;86(2):113–129. doi: 10.1016/s0165-1781(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Flyckt L, Wiesel FA, Borg J, Edman G, Ansved T, Sydow O, Borg K. Neuromuscular and psychomotor abnormalities in patients with schizophrenia and their first-degree relatives. J. Psychiatr. Res. 2000;34(4-5):355–364. doi: 10.1016/s0022-3956(00)00031-5. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Saint-Cyr JA, Weinberger DR. Assessment of procedural learning and problem solving in schizophrenic patients by Tower of Hanoi type tasks. J. Neuropsychiatry Clin. Neurosci. 1990;2(2):165–173. doi: 10.1176/jnp.2.2.165. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Williams O, McGurk S, Kee K. Procedural Learning in Schizophrenia: Evidence from Serial Reaction Time. Cognitive Neuropsychiatry. 1997;2(2):123–134. doi: 10.1080/135468097396360. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia - The consortium on the genetics of schizophrenia. Arch. Gen. Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25(5):766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J. Neurosci. Methods. 2010;187(2):254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Calkins ME, Gur RC, Horan WP, Nuechterlein KH, Seidman LJ, Stone WS. The consortium on the genetics of schizophrenia: Neurocognitive endophenotypes. Schizophr. Bull. 2007;33(1):49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Nirngaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. A. J. Psychiatry. 2007;164(5):813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Harris MSH, Wiseman CL, Reilly JL, Keshavan MS, Sweeney JA. Effects of Risperidone on Procedural Learning in Antipsychotic-Naive First-Episode Schizophrenia. Neuropsychopharmacology. 2009;34(2):468–476. doi: 10.1038/npp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur. J. Neurosci. 2008;28(6):1216–1221. doi: 10.1111/j.1460-9568.2008.06421.x. [DOI] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, Maertens de Noordhout A, Moonen G, Maquet P. Early boost and slow consolidation in motor skill learning. Learn. Mem. 2006;13(5):580–583. doi: 10.1101/lm.239406. [DOI] [PubMed] [Google Scholar]
- Jancke J, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner HW. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cognitive Brain Research. 1998;6(4):279–284. doi: 10.1016/s0926-6410(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Jancke L, Lutz K, Koeneke S. Converging evidence of ERD/ERS and BOLD responses in motor control research. Event-Related Dynamics of Brain Oscillations. 2006;159:261–271. doi: 10.1016/S0079-6123(06)59018-1. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, Calleja M, Alonso-Navarro H, Rubio L, Navacerrada F, Pilo-de-la-Fuente B, Plaza-Nieto JF, Arroyo-Solera M, Garcia-Ruiz PJ, Garcia-Martin E, Agundez JA. Influence of age and gender in motor performance in healthy subjects. J. Neurol. Sci. 2011;302(1-2):72–80. doi: 10.1016/j.jns.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Marshall BD, Jr., Wirshing WC, Wirshing D, McGurk S, Marder SR, Mintz J. Risperidone vs. haloperidol on reaction time, manual dexterity, and motor learning in treatment-resistant schizophrenia patients. Biol. Psychiatry. 1998;44(8):726–732. doi: 10.1016/s0006-3223(98)00088-2. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Battista C, Jancke L, Peters M. Transfer Effects of Practice for Simple Alternating Movements. Journal of Motor Behavior. 2009;41(4):347–355. doi: 10.3200/JMBR.41.4.347-356. [DOI] [PubMed] [Google Scholar]
- Kumari V, Corr PJ, Mulligan OF, Cotter PA, Checkley SA, Gray JA. Effects of acute administration of d-amphetamine and haloperidol on procedural learning in man. Psychopharmacology (Berl) 1997;129(3):271–276. doi: 10.1007/s002130050190. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SCR, Ng VW, Vythelingum GN, Simmons A, Suckling J, Corr PJ, Sharma T. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr. Res. 2002;57(1):97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol. Psychiatry. 2004;56(12):951–956. doi: 10.1016/j.biopsych.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Tahkkar KN, Stroynowski E, Ely A, McKinley SK, Wamsley E, Djonlagic I, Vangel MG, Goff DC, Stickgold R. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J. Psychiatr. Res. 2010;44(2):112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CH, Haughton VM, Cordes D, Quigley M, Meyerand ME. Whole-brain functional MR imaging activation from a finger-tapping task examined with independent component analysis. American Journal of Neuroradiology. 2000;21(9):1629–1635. [PMC free article] [PubMed] [Google Scholar]
- Nalçaci E, Kalaycioglu C, Cicek M, Genc Y. The relationship between handedness and fine motor performance. Cortex. 2001;37(4):493–500. doi: 10.1016/s0010-9452(08)70589-6. [DOI] [PubMed] [Google Scholar]
- Paquet E, Soucy JP, Stip E, Levesque M, Elie A, Bedard MA. Comparison between olanzapine and haloperidol on procedural learning and the relationship with striatal D-2 receptor occupancy in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2004;16(1):47–56. doi: 10.1176/jnp.16.1.47. [DOI] [PubMed] [Google Scholar]
- Peters M. Why the Preferred Hand Taps More Quickly Than the Non-Preferred Hand - 3 Experiments on Handedness. Canadian Journal of Psychology-Revue Canadienne De Psychologie. 1980;34(1):62–71. [Google Scholar]
- Peters M, Durding B. Left-handers and Right-handers Compared on a Motor Task. Journal of Motor Behavior. 1979;11(2):103–111. doi: 10.1080/00222895.1979.10735178. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Waldie B, Woodward ND, Wilman AH, Tibbo PG. Procedural learning in first episode schizophrenia investigated with functional magnetic resonance imaging. Neuropsychology. 2011;25(2):147–158. doi: 10.1037/a0021222. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology (Berl) 2003;169(3-4):390–397. doi: 10.1007/s00213-003-1505-z. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Woodward ND, Mintz A, LaBelle A. Procedural learning improvements after six weeks of clozapine treatment. Schizophr. Res. 2002;53(1-2):165–166. doi: 10.1016/s0920-9964(00)00193-6. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bandettini PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS. Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J. Cereb. Blood Flow Metab. 1996;16(6):1250–1254. doi: 10.1097/00004647-199611000-00020. [DOI] [PubMed] [Google Scholar]
- Remillard S, Pourcher E, Cohen H. Long-term skill proceduralization in schizophrenia. J. Int. Neuropsychol. Soc. 2010;16(1):148–156. doi: 10.1017/S1355617709991123. [DOI] [PubMed] [Google Scholar]
- Sadato N, Ibanez V, Campbell G, Deiber MP, LeBihan D, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements: Functional MRI compared to PET. J. Cereb. Blood Flow Metab. 1997;17(6):670–679. doi: 10.1097/00004647-199706000-00008. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . 9.22 User’s Guide. SAS Institute Inc; Cary, NC: 2010. [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Shtasel DL, Flannery KA, Mozley LH, Malamut BL, Watson B, Mozley PD. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Applied Neuropsychology. 1995;2(2):79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- Scherer H, Bedard MA, Stip E, Paquet F, Richer F, Beriault M, Rodriguez JP, Motard JP. Procedural learning in schizophrenia can reflect the pharmacologic properties of the antipsychotic treatments. Cogn Behav Neurol. 2004;17(1):32–40. doi: 10.1097/00146965-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Scherer H, Stip E, Paquet F, Bedard MA. Mild procedural learning disturbances in neuroleptic-naive patients with schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2003;15(1):58–63. doi: 10.1176/jnp.15.1.58. [DOI] [PubMed] [Google Scholar]
- Schmidt SL, Oliveira RM, Krahe TE, Filgueiras CC. The effects of hand preference and gender on finger tapping performance asymmetry by the use of an infra-red light measurement device. Neuropsychologia. 2000;38(5):529–534. doi: 10.1016/s0028-3932(99)00120-7. [DOI] [PubMed] [Google Scholar]
- Schroder J, Essig M, Baudendistel K, Jahn T, Gerdsen I, Stockert A, Schad LR, Knopp MV. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: A study with functional magnetic resonance imaging. Neuroimage. 1999;9(1):81–87. doi: 10.1006/nimg.1998.0387. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, Veazey C, Deutsch SI. Impaired motor skill learning in schizophrenia: Implications for corticostriatal dysfunction. Biol. Psychiatry. 1996;39(4):241–248. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Regan V. Sequencing, timing, and rate relationships between language and motor skill in children with receptive language delay. Developmental Neuropsychology. 1996;12(3):255–270. [Google Scholar]
- Shimoyama I, Ninchoji T, Uemura K. The Finger-Tapping Test - a Quantitative-Analysis. Arch. Neurol. 1990;47(6):681–684. doi: 10.1001/archneur.1990.00530060095025. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Weatherall M, Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain Cognition. 2008;67(3):351–359. doi: 10.1016/j.bandc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Squire LR. Mechanisms of memory. Science. 1986;232(4758):1612–1619. doi: 10.1126/science.3086978. [DOI] [PubMed] [Google Scholar]
- Stip E. Encephale-Revue De Psychiatrie Clinique Biologique Et Therapeutique. 2006;32(3 Pt 1):341–350. doi: 10.1016/s0013-7006(06)76162-0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. A. J. Psychiatry. 1998;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- Witt ST, Laird AR, Meyerand ME. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. Neuroimage. 2008;42(1):343–356. doi: 10.1016/j.neuroimage.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfinger R, Ming C. Comparing the SAS® GLM and MIXED Procedures for Repeated Measures; Paper presented at the Proceedings of the Twentieth Annual SAS Users Group Conference; 1995. [Google Scholar]
- Woodward ND, Tibbo P, Purdon SE. An fMRI investigation of procedural learning in unaffected siblings of individuals with schizophrenia. Schizophr. Res. 2007;94(1-3):306–316. doi: 10.1016/j.schres.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Zedkova L, Woodward ND, Harding I, Tibbo PG, Purdon SE. Procedural learning in schizophrenia investigated with functional magnetic resonance imaging. Schizophr. Res. 2006;88(1-3):198–207. doi: 10.1016/j.schres.2006.06.039. [DOI] [PubMed] [Google Scholar]