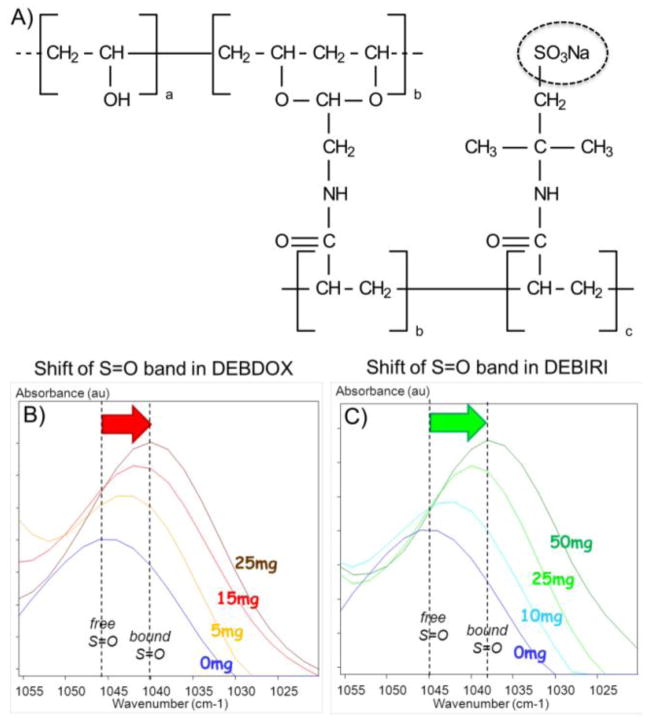
Figure 3.
A) Chemical structure of the sulfonate-modified polyvinyl alcohol hydrogel polymer used in the fabrication of DC Bead. B) and C) Demonstration of the ionic interaction between the sulfonate groups in DEB (see dashed circle in A) with doxorubicin (B) or irinotecan (C) using Fourier Transform Infrared Microscopy. Note the shift in the position of the S=O stretching absorption from higher to lower wavenumber with increasing concentration of drug bound within the system, denoting a shift from free to bound S=O groups. B and C courtesy of Dr. J. Namur.