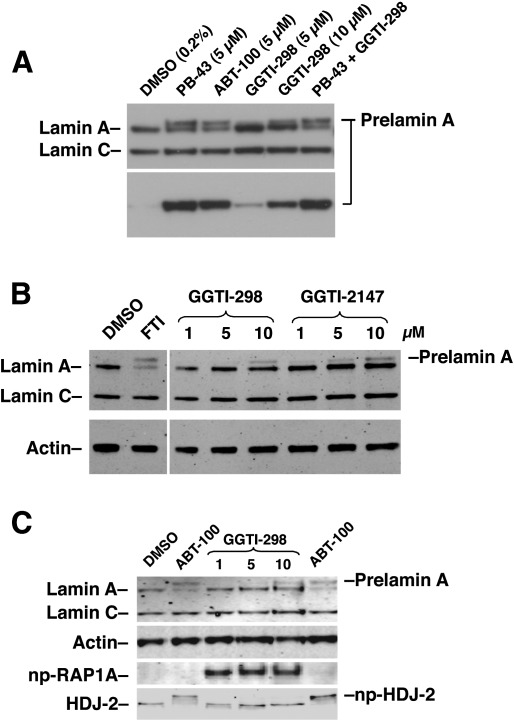
Fig. 1.
Treatment of human fibroblasts with a GGTI leads to an accumulation of prelamin A. (A) Western blot of protein extracts from human fibroblasts treated with an FTI (PB-43 or ABT-100) or a GGTI (GGTI-298) at the concentrations indicated, or with both PB-43 (5 μM) and GGTI-298 (10 μM). Prelamin A accumulation was detected with both a lamin A/C antibody (top panel) and a prelamin A–specific antibody (bottom panel). (B) Western blots, with antibodies against lamin A/C and actin, of protein extracts from human fibroblasts treated with an FTI or two different GGTIs (GGTI-298 or GGTI-2147) in increasing concentrations. (C) Western blots of protein extracts from fibroblasts treated with an FTI (ABT-100) or a GGTI (GGTI-298). As expected, only the FTI, and not the GGTI, inhibited the farnesylation of HDJ-2 (np-HDJ-2). Also, only the GGTI, and not the FTI, led to an accumulation of the nonprenylated version of RAP1A (np-RAP1A). Both the FTI and GGTI-298 led to prelamin A accumulation. In all three Western blots, the prelamin A in the GGTI-treated cells migrated slightly more rapidly than the prelamin A in FTI-treated cells.