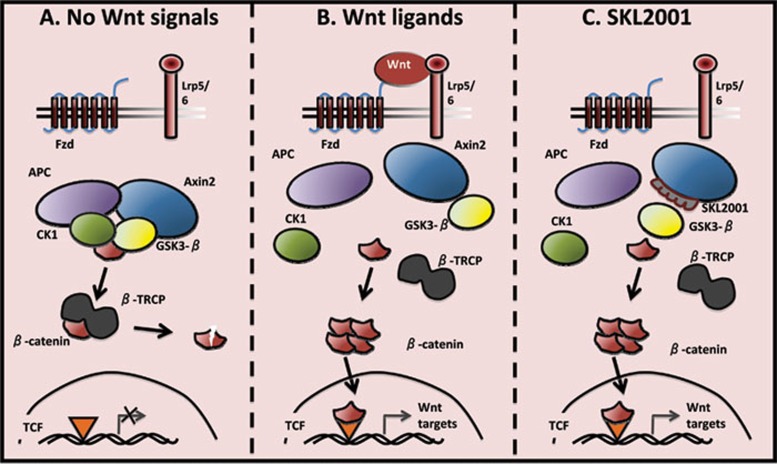
Figure 1.
(A) In the absence of Wnt ligands, β-catenin is kept under low cytosolic level by the destruction complex. This complex contains Axin2 and APC, which present β-catenin to the 2 kinases CK1 and GSK3-β, enabling its phosphorylation at specific serine and threonine residues. This primes β-catenin recognition by β-TRCP, which targets it for proteosomal degradation. In the nucleus, TCF transcription factors are repressed and Wnt target gene transcription is inhibited. (B) When Wnt ligands bind to Fzd and LRP5/6 co-receptors, the destruction complex is dissolved. β-catenin is stabilized, can accumulate in the cytosol, and subsequently, translocate into the nucleus where it converts TCF into a transcriptional activator. These events trigger an efficient transcription of genes that are important regulators of stem cell fate, cell proliferation as well as cell fate determination. (C) The small molecule agonist SKL2001 binds to Axin2 and precludes its interaction with GSK3-β, leading to an inefficient targeting of β-catenin for degradation. Therefore, β-catenin can accumulate and form active transcription factor complexes with TCF proteins in the nucleus, even in the absence of external signal.