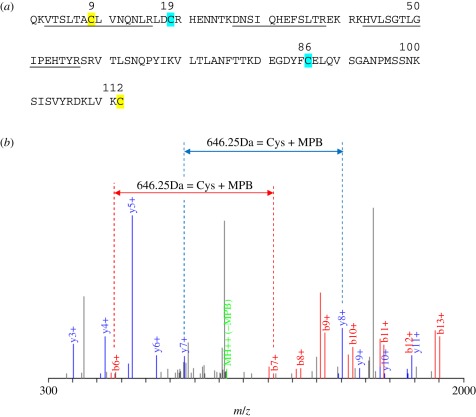
Figure 2.
Analysis of Thy-1 isolated after reduction with TCEP showing peptide coverage and MBP-modified peptide. (a) Amino acid sequence of mouse Thy-1 showing the peptides identified by mass spectrometry (underlined) and the peptide containing the biotin–maleimide modification (residue 9; yellow), which forms a labile disulfide bond with the Cys (112; yellow) at the C-terminus. Cys (112) would not be expected to be recognized by MS as the predicted tryptic peptide is a single residue that is coupled to the glycophosphatidylinositol anchor. The Cys residues for the other stable disulfide (Cys19 and Cys86) are shown in blue. (b). The MS/MS spectrum of peptide VTSLTAC(MPB)LVNQNLR shows good unambiguous coverage of the b+ (red peaks) and y+ (blue peaks) ion series. Sequential individual amino acid masses were identified in both the b+ and y+ ions series except for Cys-7, which has the MPB tag attached. A mass difference of 646.25 kDa between b6+–b7+ (red dashed lines) and y7+–y8+ (blue dashed lines) corresponds to the mass of Cys + MPB.