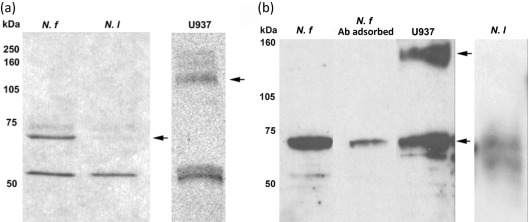
Fig. 4.
Western immunoblot analysis of (a) N. fowleri (N. f) and N. lovaniensis (N. l) whole-cell lysates and (b) N. fowleri and N. lovaniensis membrane fractions for the detection of integrin-like proteins. Amoebic extracts were separated by 12 % SDS-PAGE and transferred to a nitrocellulose membrane (left panel). A human leukaemic monocyte lymphoma (U937) cell extract was used as a positive integrin control. Membranes then were incubated with a polyclonal chicken anti-human β1 integrin subunit antibody followed by horseradish peroxidase-conjugated rabbit anti-chicken antibody. The chicken polyclonal antibody directed against the human β1 integrin subunit used in Western immunoblotting was pre-adsorbed with U937 cells prior to use to serve as a negative control (N. f Ab adsorbed). Arrows indicate the 70 kDa (N. fowleri and N. lovaniensis) and 140 kDa (U937) major immunoreactive bands.