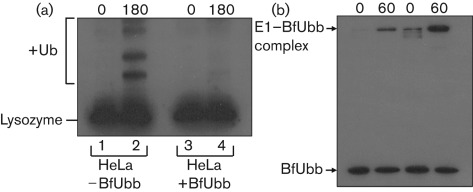
Fig. 4.
In vitro activity of BfUbb. (a) Immunoblot of in vitro ubiquitination of lysozyme using HeLa cell extract in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of BfUbb. Samples were taken at the start of the reaction (0) and at 180 min (180). Lane 2 shows the increase in molecular mass of the lysozyme substrate following covalent attachment of ubiquitin (+Ub). This covalent modification is inhibited by the addition of BfUbb (lane 4). (b) Covalent complexes between human E1 and BfUbb, under non-reducing conditions, were detected by immunoblotting using anti-bovine ubiquitin polyclonal serum. Two reactions using different concentrations of E1 are shown, with time points at 0 and 60 min. Note there was some antibody cross-reactivity with E1 at the higher concentration.