Abstract
Herpesvirus saimiri (HVS), a T-lymphotropic monkey herpesvirus, induces fulminant T-cell lymphoma in non-natural primate hosts. In addition, it can immortalize human T-cells in vitro. HVS tyrosine kinase-interacting protein (Tip) is an essential viral gene required for T-cell transformation both in vitro and in vivo. In this study, we found that Tip interacts with the STAT6 transcription factor and induces phosphorylation of STAT6 in T-cells. The interaction with STAT6 requires the Tyr127 residue and Lck-binding domain of Tip, which are indispensable for interleukin (IL)-2-independent T-cell transformation by HVS. It was also demonstrated that Tip induces nuclear translocation of STAT6, as well as activation of STAT6-dependent transcription in Jurkat T-cells. Interestingly, the phosphorylated STAT6 mainly colocalized with vesicles containing Tip within T-cells, but was barely detectable in the nucleus. However, nuclear translocation of phospho-STAT6 and transcriptional activation of STAT6 by IL-4 stimulation were not affected significantly in T-cells expressing Tip. Collectively, these findings suggest that the constitutive activation of STAT6 by Tip in T-cells may contribute to IL-2-independent T-cell transformation by HVS.
Introduction
Herpesviruses persist in their hosts by entering a latent state and reactivating periodically to produce infectious virus particles. Herpesvirus saimiri (HVS), an oncogenic γ2 herpesvirus, persists in the T-lymphocytes of its natural host, squirrel monkey, without any apparent disease symptoms, but infection of other New World and Old World primate species results in fulminant T-cell lymphoma (Jung et al., 1999). In addition, when HVS infects the primary T-lymphocytes of humans, Old World primates, New World primates or rabbits in vitro, it can immortalize infected T-cells, allowing them to grow independently of IL-2 (Biesinger et al., 1992).
Tyrosine kinase-interacting protein (Tip) is encoded in the first ORF at the left end of the highly oncogenic strains of HVS. Although it is not required for virus replication, Tip is required for T-cell transformation in cultures and for lymphoma induction in primates (Jung et al., 1999). Tip has multiple binding sites for cellular proteins. The interaction of Tip with Lck kinase, which is mediated by the Src homology 3-binding (SH3B) motif and C-terminal Src-related kinase homology (CSKH) domain of Tip (Hartley et al., 2000; Jung et al., 1995), interferes with early events in the T-cell receptor (TCR) signal-transduction pathway, resulting in inhibition of immunological-synapse formation (Cho et al., 2004). Tip also interacts with p80, a cellular endosomal protein that contains an N-terminal WD-repeat domain and a C-terminal coiled-coil domain (Park et al., 2002). The interaction of Tip with p80, which is mediated by a region containing a serine-rich (SR) motif, facilitates the formation of enlarged lysosomal vesicles and results in the targeting of Lck and TCR–CD3 complexes for lysosomal degradation. It has been demonstrated previously that Tip constitutively localizes in lipid rafts and exploits Lck and p80 to recruit TCR–CD3 complexes, leading to lipid-raft aggregation and internalization (Cho et al., 2006; Park et al., 2003). Constitutive localization of Tip in lipid rafts depends on its C-terminal transmembrane (TM) domain, but not Lck and p80 interaction, and is also necessary for the efficient downregulation of TCR–CD3 and CD4 surface expression without affecting the inhibition of TCR signal transduction (Cho et al., 2006). Recently, it was also reported that the membrane-proximal amphipathic helix preceding Tip’s TM domain mediates lipid-raft localization and membrane deformation (Min et al., 2008). In turn, this motif directs Tip’s lysosomal trafficking and selective TCR downregulation. The amphipathic helix of Tip binds to the negatively charged lipids and induces liposome tabulation, whilst its TM domain mediates oligomerization. Moreover, cooperation of the membrane-proximal helix with the TM domain is sufficient for the localization of Tip to lipid rafts and lysosomal compartments, especially the mutivesicular bodies.
In addition to Lck and p80, Tip has been shown to interact with signal transducers and activators of transcription (STATs) and to bind to phosphorylated STAT1 and STAT3 together with Lck (Heck et al., 2005; Lund et al., 1997). A YXPQ motif of Tip conforms to a putative binding site for STAT factors (Shao et al., 2004), and phosphorylation at Tyr114 in the C488 strain, equivalent to Tyr72 in the C484 strain, is required for STAT binding and transcriptional activation (Cho et al., 2006; Hartley & Cooper, 2000). STATs are key mediators involved in signalling by various cytokines, including the interleukin (IL)-6 and IL-2 family cytokines, as well as numerous growth factors (Levy & Darnell, 2002). STATs reside in the cytoplasm during unstimulated conditions and, upon stimulation, are recruited to a cytokine receptor where they are tyrosine-phosphorylated. Active STAT dimers are then formed via the reciprocal interaction between their SH2 domains. Subsequently, they translocate to the nucleus, where they bind to specific DNA-response elements in the promoters of target genes to activate transcription. Furthermore, various oncoproteins can activate specific STAT molecules, and inappropriate STAT activation contributes directly to oncogenesis by stimulating cell proliferation and preventing apoptosis (Haura et al., 2005). Constitutive activation of several STATs was detected frequently in a wide range of human cell lines and primary tumours, including lymphoid malignancies (Haura et al., 2005; Yu et al., 2009). Recently, however, HVS carrying a mutant Tip in which Tyr114 was changed into phenylalanine was still able to transform human T-lymphocytes, while losing its capability to activate STAT3 as well as STAT1 (Heck et al., 2005). This result demonstrated that growth transformation by HVS is independent of STAT3 activation. Interestingly, recombinant virus expressing Tip with a mutation at Tyr127 was still capable of transforming human T-lymphocytes but, in contrast to the wild type (wt), was strictly dependent on exogenous IL-2 (Heck et al., 2006). The Tyr127 of Tip was particularly required for transformation in the absence of exogenous IL-2, suggesting its involvement in cytokine signalling pathways.
Based on the significance of Tyr127 of Tip on IL-2-independent transformation, we aimed to search for a novel binding target of the tyrosine residue to reveal the basic mechanisms by which HVS Tip can contribute to IL-2-independent transformation of human T-cells. Here, we show that the Tyr127 residue of Tip is required for the interaction with STAT6 after phosphorylation, leading to nuclear translocation and transcriptional activation of STAT6 and, ultimately, IL-2-independent transformation of T-cells by HVS.
Results
HVS Tip interacts with the STAT6 transcription factor
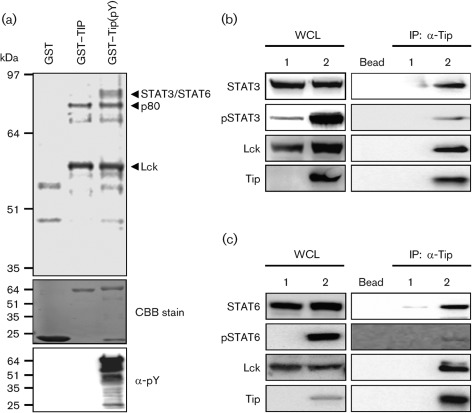
In order to identify host proteins interacting with Tip, a glutathione S-transferase (GST) pull-down assay was performed using bacterially produced GST-fusion proteins. Unphosphorylated GST–Tip containing the cytoplasmic region of Tip was produced from Escherichia coli strain BL21(DE3), and tyrosine-phosphorylated GST–Tip(pY) was purified from E. coli strain TKX1, containing the elk tyrosine kinase, which has broad specificity and phosphorylates mammalian proteins efficiently in E. coli. We found that the GST–Tip protein purified from E. coli TKX1 was tyrosine-phosphorylated efficiently by elk kinase (Fig. 1a). To identify cellular proteins interacting with Tip in a phosphorylation-dependent manner, the bacterially purified GST–Tip and GST–Tip(pY) fusion proteins were used on an affinity column for 35S-labelled lysates of Jurkat cells. Polypeptides with apparent molecular masses of 56 and 80 kDa interacted specifically with GST–Tip, whereas they did not interact with the GST protein (Fig. 1a). Furthermore, polypeptides with a molecular mass of 90 kDa interacted specifically with the GST–Tip(pY) fusion protein, but did not interact with the GST and GST–Tip fusion proteins (Fig. 1a). To characterize these cellular proteins further, they were analysed by mass spectrometry and matched with known sequences. The two cellular proteins that interacted with both GST–Tip and GST–Tip(pY) were the Lck and p80 proteins, which are the known cellular targets of Tip (Jung et al., 1995; Park et al., 2002). The cellular proteins with a molecular mass of 90 kDa that interacted only with the tyrosine-phosphorylated GST–Tip(pY) protein but not with the unphosphorylated GST–Tip protein were identified as the STAT3 and STAT6 transcription factors (Fig. 1a), indicating that these transcription factors interact with Tip in a phosphorylation-dependent manner.
Fig. 1.
Tip interacts with STAT6. (a) To identify the proteins binding to Tip, a GST pull-down assay was performed with GST-fused phosphorylated Tip purified from E. coli strain TKX1 and Jurkat T-cell lysate. Expression of GST-tagged proteins and formation of phosphorylated protein were visualized by Coomassie brilliant blue (CBB) staining and immunoblotting using an anti-phosphotyrosine antibody (α-pY), respectively. Binding proteins were analysed by mass spectrometry. To confirm the interaction between Tip and STAT proteins, HEK293T cells were transfected with Lck and (b) STAT3 or (c) STAT6, together with (lane 2) or without (lane 1) Tip. Lysates were immunoprecipitated using anti-Tip antibody and immunocomplexes were analysed by immunoblotting using the indicated antibodies. Protein A/G beads without antibody were used to exclude non-specific binding (Bead). IP, Immunoprecipitation; WCL, whole-cell lysate.
Previous reports have shown that Tip binds directly to STAT3 and activates the transcription factor through tyrosine phosphorylation in the presence of Lck (Hartley & Cooper, 2000; Lund et al., 1997). To confirm the functional interaction of Tip with STAT6 in addition to STAT3, 293T cells were electroporated with plasmids encoding Tip and its cellular partners. Tip was precipitated from these cells and an immunoblot analysis was performed to test the complex formation and tyrosine phosphorylation of the STAT transcription factors. As shown in Fig. 1(b, c), rigorous phosphorylation of STAT6 as well as STAT3 was detected in the presence of Tip, and both transcription factors were present in Tip–Lck complexes.
Tip induces nuclear translocation and activation of STAT6 in Jurkat T-cells
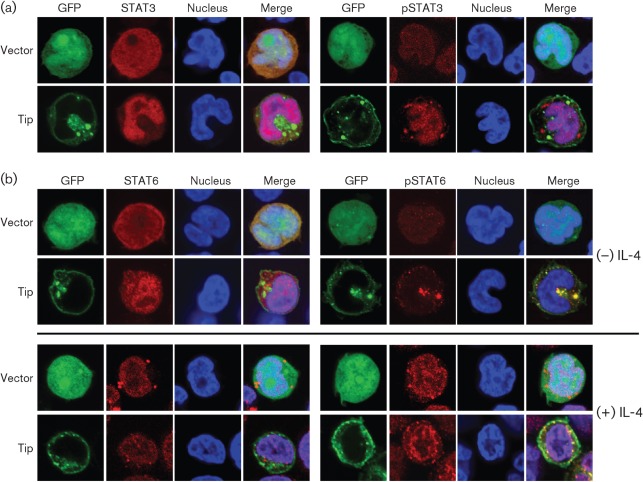
As STAT transcription factors are known to be translocated into the nucleus after being activated by phosphorylation, we next examined the effect of Tip on STAT6 localization. Jurkat T-cells electroporated with a plasmid encoding GFP (vector) or GFP–Tip were monitored using confocal immunofluorescence. As reported previously (Cho et al., 2006), STAT3 was translocated efficiently into the nucleus in cells expressing GFP–Tip, whereas this transcription factor was detected throughout cells expressing GFP (Fig. 2a). In addition, phosphorylated STAT3 was primarily present in the nucleus, indicating that the induced phosphorylation of STAT3 by Tip enhanced nuclear translocation of the transcription factor. When we examined STAT6 in Jurkat T-cells expressing Tip, it also showed an increase in nuclear translocation compared with control cells (Fig. 2b, upper panels). Unexpectedly, however, phosphorylated STAT6 colocalized primarily with Tip complexes located within the cytoplasm rather than the nucleus. To investigate further the role of Tip in the localization of STAT6, especially its phosphorylated form, we treated Jurkat T-cells with IL-4, which is a well-known cytokine inducing activation of STAT6 (Yu et al., 2009). Fifteen minutes after IL-4 treatment, STAT6 was translocated efficiently into the nucleus in Jurkat T-cells expressing GFP or GFP–Tip (Fig. 2b, lower panels). Phosphorylated STAT6 was also detected primarily in the nucleus in IL-4-treated cells, regardless of Tip expression, indicating that Tip does not significantly affect the nuclear translocation of STAT6 in response to IL-4. To demonstrate further that the Tip-mediated phosphorylation and nuclear translocation of STAT6 lead to transcriptional activity of STAT6, Jurkat T-cells were electroporated with a STAT6-responsive luciferase reporter plasmid together with GFP vector or GFP–Tip plasmid. As shown in Fig. 3, Tip expression enhanced STAT6 transcriptional activity strongly in Jurkat T-cells compared with control cells expressing GFP (vector). The level of induction of STAT6 activity by Tip was comparable to that of control cells stimulated by IL-4, and there was only a slight increase in STAT6 activity in cells expressing Tip upon IL-4 treatment. The results further showed that Tip could dramatically induce the activation of STAT6 without exogenous stimulation in Jurkat T-cells.
Fig. 2.
Tip induces nuclear translocation of STAT6. Jurkat T-cells were electroporated with plasmids encoding GFP (vector) or GFP–Tip together with pVR/STAT3 or STAT6. Twenty-four hours after electroporation, localization of STAT3 (a) and STAT6 (b) was examined by confocal microscopy after staining with anti-STAT or anti-phosphoSTAT antibodes (red). TO-PRO-3 staining was used to visualize the nucleus (blue). Nuclear translocation of STAT6 was also examined in the absence (−) or presence (+) of IL-4 treatment (100 ng ml−1, 15 min).
Fig. 3.
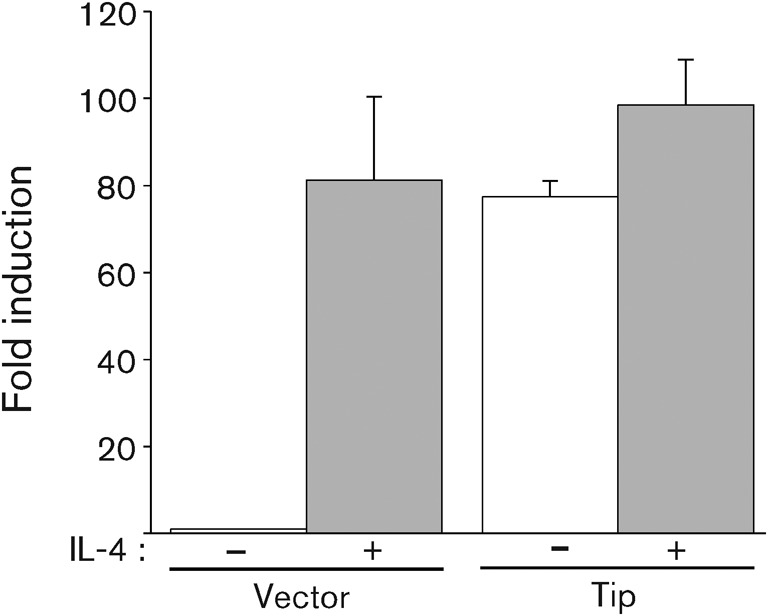
Tip induces transcriptional activation of STAT6. Jurkat T-cells were electroporated with plasmids encoding Tip, STAT6 and 3×STAT6-luc plasmid and cultured in the presence (grey) or absence (white) of IL-4 (20 ng ml−1) for 18 h. To normalize transfection efficiency, pGK-βgal vector was included in the transfection mixture and fold induction of luciferase activity was determined after normalization with β-galactosidase activity. Each bar represents data from triplicate assays; error bars indicate sd.
Tyr127 of Tip is required for STAT6 interaction and activation
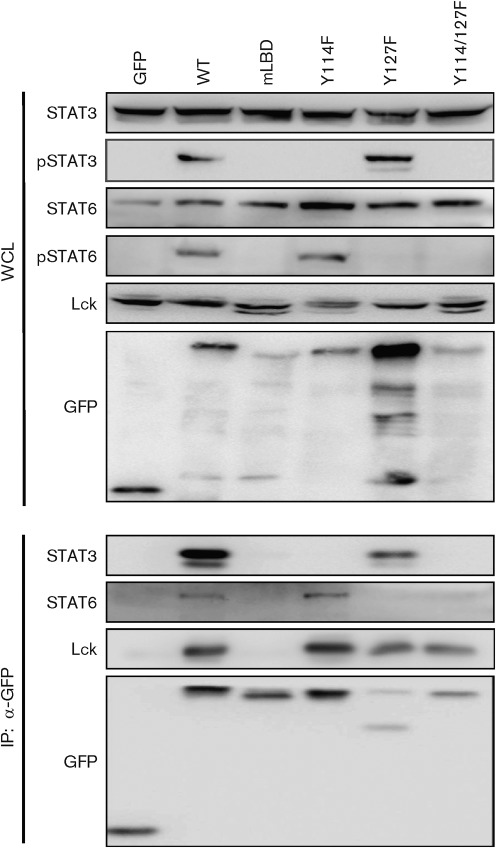
Previously, it has been demonstrated that Tip becomes tyrosine-phosphorylated by Lck at two sites and that one (Y72 of Tip-C484 and Y114 of Tip-C488) of the tyrosine residues embedded in a STAT SH2-binding motif (YXPQ) is required for direct interaction with, and thus activation of, STAT1 and STAT3 in the presence of Lck (Hartley & Cooper, 2000; Kjellen et al., 2002; Lund et al., 1997, 1999). In order to examine whether tyrosine phosphorylation of Tip is required for the binding of STAT6 and induction of STAT6 phosphorylation, Jurkat T-cells were electroporated with plasmids encoding Tip wt or its mutants and subjected to immunoprecipitation. As seen in Fig. 4, STAT3 was tyrosine-phosphorylated and co-precipitated with Tip in cells expressing Tip wt and the Y127F mutant, whereas expression of the Y114F or Lck-binding (mLBD) mutants of Tip failed to induce tyrosine phosphorylation of STAT3 and the inclusion of STAT3 into a Tip–Lck complex, as expected. However, when the Y114F mutant was expressed, there was an increase in the tyrosine phosphorylation of STAT6, which correlated with the immune-complex formation with Tip and Lck. In contrast, the Y127F and mLBD mutants did not induce phosphorylation of STAT6 and failed to interact with STAT6. These results indicate that Tyr127 in Tip is required for STAT6 binding and phosphorylation, whereas Tyr114 serves as a docking site for STAT3 for its phosphorylation.
Fig. 4.
The Tyr127 residue of Tip is required for the interaction with and phosphorylation of STAT6. Jurkat T-cells were electroporated with GFP, GFP–Tip or GFP–Tip mutant expression constructs. Twenty-four hours after electroporation, cells were lysed and used for immunoprecipitation (IP) with an antibody against GFP, followed by immunoblotting with the indicated antibodies. Whole-cell lysates (WCL) were used to detect the expression level of STATs and Lck.
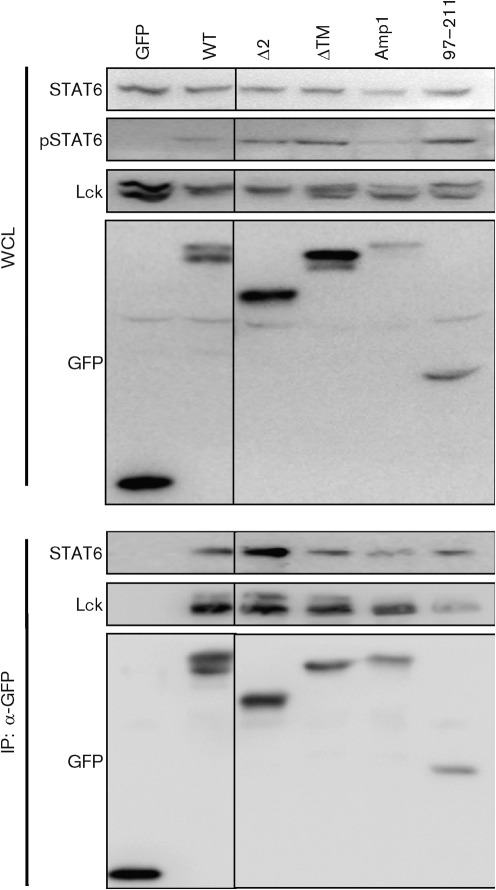
We have demonstrated previously that Tip is targeted constitutively to lipid rafts, membrane microdomains that function as platforms for modulating both TCR signalling and trafficking (Cho et al., 2004, 2006; Min et al., 2008; Park et al., 2002). This functional association of Tip with lipid rafts is dependent on multiple sequence motifs, including the p80-binding domain (Park et al., 2003), transmembrane domain (Cho et al., 2006) and membrane-proximal amphipathic helix (Min et al., 2008). To test whether the association of Tip with lipid rafts affects the activation of STAT6, tyrosine phosphorylation of STAT6 and its complex formation with Tip–Lck were examined in Jurkat T-cells expressing Tip wt or its mutants that fail to associate with lipid rafts. As shown in Fig. 5, all tested mutants induced tyrosine phosphorylation of STAT6 and interacted with the transcription factor as efficiently as Tip wt. Even the 97–211 fragment mutant, which has extensive deletions in both the N and C termini, induced STAT6 phosphorylation sufficiently and interacted with the transcription factor.
Fig. 5.
Association of Tip with membrane lipid rafts is not required for its interaction with and phosphorylation of STAT6. Jurkat T-cells were electroporated with plasmids encoding Tip or its mutants that are defective in lipid-raft association or membrane trafficking. Twenty-four hours after electroporation, cells were lysed and used for immunoprecipitation (IP) with an antibody against GFP. Whole-cell lysates (WCL) and immunocomplexes were subjected to immunoblot assay using the indicated antibodies.
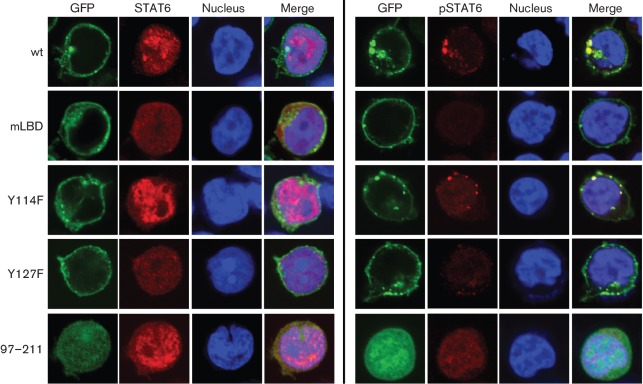
To further confirm the role of Tyr127 of Tip in STAT6 activation, we examined nuclear translocation of the transcription factors using the Tip mutants. Jurkat T-cells were electroporated with plasmids encoding GFP–Tip wt or its mutants for confocal microscopy analysis. STAT6 was translocated efficiently into the nucleus upon expression of Tip wt and the Y114F and 92–211 mutants, whereas expression of Y127F or mLBD did not induce nuclear localization of STAT6 under the same conditions (Fig. 6). Consistently, phosphorylated STAT6 was detected primarily in association with the Tip complex within the cytosol in cells expressing Tip wt or Y114F. In contrast, it was barely detected in Jurkat T-cells expressing the mLBD or Y127F mutants. Interestingly, phosphorylated STAT6 was observed within the nucleus in cells expressing the 97–211 mutant, incapable of associating with lipid rafts or membranous vesicles (Fig. 6). The role of the Tyr127 residue in the activation of STAT6 transcription-factor activity was also tested using a STAT6-responsive reporter system. The Tip Y127F mutant exhibited no effect on STAT6 transcriptional activity, whereas the Y114F mutant induced STAT6 activation as proficiently as Tip wt (Fig. 7). In addition, the 97–211 deletion mutant showed a remarkable increase in STAT6 transcriptional activity. Taken together, these results indicate clearly that the Tyr127 residue is essential for the interaction of Tip with STAT6 and is required for the activation of STAT6 transcriptional activity.
Fig. 6.
The Tyr127 residue of Tip is required for nuclear translocation of STAT6 by Tip. Jurkat T-cells were electroporated with plasmids encoding GFP-fused Tip wt or its mutants (green) together with pVR/STAT6. Twenty-four hours after electroporation, localization of STAT6 (left panels) and phosphoSTAT6 (right panels) was visualized by confocal microscopy after staining with anti-STAT6 or anti-phosphoSTAT6 antibodies (red). TO-PRO-3 staining was used to visualize the nucleus (blue).
Fig. 7.
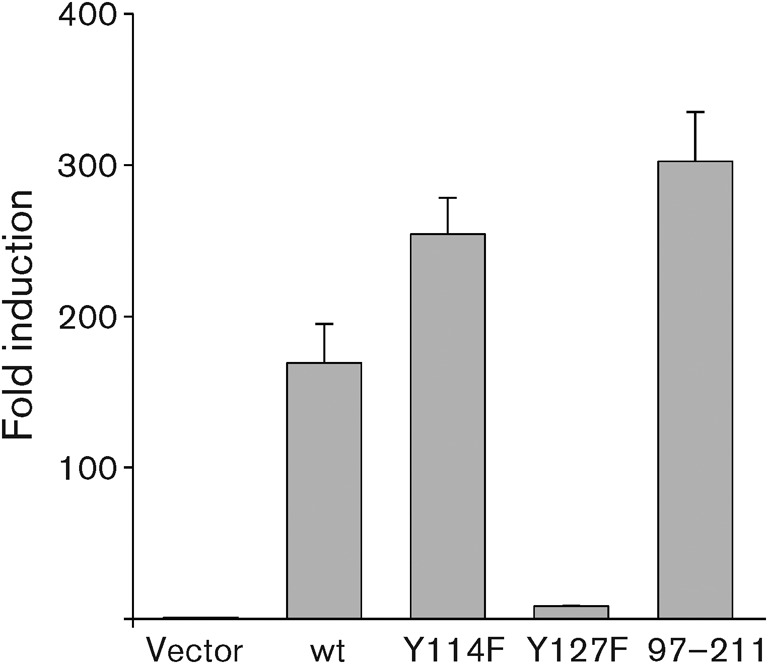
The Tyr127 residue of Tip is required for transcriptional activation of STAT6. Jurkat T-cells were electroporated with plasmids encoding Tip wt or its mutants together with pVR/STAT6 and 3×STAT6-luc plasmid. Twenty-four hours after electroporation, luciferase activities were measured. To normalize transfection efficiency, the pGK-βgal vector was included in the transfection mixture and fold induction of luciferase activity was determined after normalization with β-galactosidase activity. Each bar represents data from triplicate assays; error bars indicate sd.
Discussion
The genome of the highly oncogenic HVS subgroup C strain contains one bicistronic gene with two ORFs, tip and stpC, which are essential for T-cell transformation. Deletions affecting the ORFs for StpC and/or Tip within the viral genome of subgroup C strains 484 or 488 abolished transformation in vitro and pathogenicity in vivo (Duboise et al., 1998; Heck et al., 2006). Independent of the viral context, both viral oncoproteins induced tumours in transgenic mice: expression of StpC resulted in epithelial hyperplasia, whilst Tip caused peripheral T-cell lymphoma closely resembling the tumours in susceptible New World primates (Heck et al., 2006; Murphy et al., 1994; Wehner et al., 2001). The effects of StpC are assigned to its interaction with the ubiquitous cellular oncoprotein Ras and to its ability to activate the transcription factor NF-κB (nuclear factor kappa B) (Choi et al., 2000; Lee et al., 1999). In accordance with the phenotype of T-cell tumours in transgenic mice, Tip binds directly to the Src family tyrosine kinase Lck, a key regulator for T-cell activation (Biesinger et al., 1995; Heck et al., 2006). The recruitment by Tip and subsequent phosphorylation by Lck provide an explanation for the observed activation of STAT1 and STAT3 in the presence of Tip and Lck (Hartley & Cooper, 2000; Lund et al., 1997, 1999). The implication of constitutively active STATs, especially STAT3, in growth regulation and oncogenesis in multiple cell types (Bowman et al., 2000; Bromberg & Darnell, 2000) suggested a central role for Tip-induced STAT activity in viral T-cell transformation. However, a recombinant HVS C488 strain expressing Tip with a tyrosine-to-phenylalanine substitution at position 114 was able to transform primary human T-lymphocytes in the absence of STAT1 or STAT3 activation (Heck et al., 2005, 2006). Thus, the essential function of Tip in lymphocyte transformation does not rely on Lck-mediated STAT1/3 phosphorylation. Previously, however, it was reported that a recombinant HVS strain C488 lacking LBD motifs of Tip lost its transforming potential on human cord-blood lymphocytes (Heck et al., 2006). In addition, a recombinant virus expressing Tip with a mutation at position Tyr127 was still able to transform human T-lymphocytes but, in contrast to wt virus, was strictly dependent on exogenous IL-2 (Heck et al., 2006). Thus, the major tyrosine-phosphorylation site Tyr127 of Tip might be required for compensating the requirement of exogenous IL-2 by constitutively activating STAT6, as shown in this study.
STATs possess SH2 domains required for interaction with proteins containing phosphotyrosine (pY). SH2 domains specifically recognize protein modules composed of pY and three to five carboxyl-terminal residues. Although each protein interacting with SH2 domain-containing proteins has a different docking-site sequence, they commonly have the motif pY-X-X-hydrophobic residue (Zhou et al., 1993). Tip has a STAT3-binding motif, Tyr114-Arg-Pro-Gln, which aligns well with the known docking sequence, Tyr-X-Pro-Gln, for STAT3 (Hartley & Cooper, 2000). Thus, phosphorylation of Tyr114 by Lck makes Tip capable of binding to STAT3 for activation (Lund et al., 1997, 1999). STAT6 also has an SH2 domain and interacts with its natural docking protein, the IL-4 receptor (IL-4R) (Hou et al., 1994). When IL-4 binds to its receptor, activated JAKs phosphorylate five tyrosine residues at Y497, Y575, Y603, Y631 and Y713 in IL-4R (Hartley & Cooper, 2000; Johnston et al., 1994; Keegan et al., 1994; Murata et al., 1996; Witthuhn et al., 1994). In particular, three conserved tyrosine residues of IL-4R, Y575, Y603 and Y631, that are critical for STAT6 binding and IL-4 signal transduction (Reichel et al., 1997; Ryan et al., 1996) are surrounded by a conserved motif, Gly-Tyr-Lys/Gln-X-Phe. In addition, Y497 of IL-4R, which is required for STAT6 binding (Ryan et al., 1996) and IL-4-mediated proliferation (Deutsch et al., 1995; Keegan et al., 1994), is also surrounded by a sequence motif, Ala-Tyr-Arg-Ser-Phe. Taken together, the common sequence, Tyr-X-X-Phe, contributes to the interaction with the SH2 domain of STAT6 after tyrosine phosphorylation. In case of Tip, the Tyr127 residue is surrounded by Tyr127-Thr-Thr-Phe, which is consistent with the general SH2 domain-binding motif in IL-4R for STAT6 binding. Furthermore, the residues (Tyr-Thr-Thr/Ser-Phe) surrounding Tip Y127 are highly conserved among different HVS subgroup C isolates (Ensser et al., 2003). Therefore, Tyr127 of Tip in the context of Tyr-X-X-Phe forms an authentic docking site for the SH2 domain of STAT6 after tyrosine phosphorylation by Lck, as shown in this study.
Constitutively active STATs and dysregulation of STAT signalling have been found in many haematocytic tumours (Benekli et al., 2003). The abnormal functions of STAT3 and STAT5 in lymphomas and leukaemias have been studied extensively in the aspect of myelopoiesis regulation in response to cytokines or hormones. Recently, the roles of other STATs have been gradually emerging for cellular transformation. STAT6 is also overactivated in Hodgkin’s lymphoma, primary mediastinal large B-cell lymphomas, cutaneous T-cell lymphomas and acute lymphoblastic leukaemia (Guiter et al., 2004; Ilaria & Van Etten, 1996; Qin et al., 2001; Skinnider et al., 2002). In these tumours, aberrant STAT6 activation occurs because of the high level of cytokines or kinases (except for JAK) or the gain-of-function mutations in STAT6.
Unexpectedly, nuclear translocation of phosphorylated STAT6 was barely detectable in T-cells expressing Tip (Fig. 2). Phosphorylated STAT6 mainly colocalized with Tip-containing vesicles in the cytoplasm. Interestingly, several reports have suggested that cytoplasmic transport of STAT3 is an active process that requires receptor-mediated endocytosis (Bild et al., 2002; Xu et al., 2007). In these reports, phosphorylated STAT3 colocalizes with endocytic vesicles in transit from the cell membrane to the perinuclear region in response to growth-factor stimulation. Consistent with a role for receptor endocytosis in growth-factor signalling, disruption of endocytosis with specific inhibitors blocks STAT3 nuclear translocation and STAT3-dependent gene regulation (Bild et al., 2002). Based on these findings and our current data, it can be suggested that cytoplasmic localization of phosphorylated STAT6 induced by Tip expression in T-cells may result from prolonged association of phosphorylated STAT6 with Tip-containing vesicles in the cytoplasm and that it eventually translocates into the nucleus and rapidly becomes dephosphorylated. Considering that Tip itself has a strong affinity for membrane lipid rafts and interacts with the cellular trafficking protein p80 for lysosomal targeting (Cho et al., 2006; Park et al., 2002, 2003), it is possible that the majority of phosphorylated STAT6 is retained in the Tip-containing trafficking vesicles during the vesicular trafficking in the cytoplasm after its phosphorylation. This hypothesis is consistent with our finding that Tip mutant 97–211, lacking the transmembrane domain and p80-binding motif but still capable of interacting with Lck and STAT6, induces rapid nuclear translocation of phosphorylated STAT6 (Fig. 6). The precise role of vesicular trafficking of the Lck–Tip–phosphoSTAT6 complex in T-cells needs to be characterized further.
Another striking observation was the dramatic increase of unphosphorylated STAT6 in the nucleus. The increased level of STAT6 in the nucleus correlates well with the transcriptional activation of a STAT6-responsive promoter in Jurkat T-cells expressing Tip to a level comparable with that of IL-4-treated cells (Fig. 3). The dramatic increase of unphosphorylated STAT6 in the nucleus might control gene expression by several different mechanisms. First, enhanced nuclear translocation of unphosphorylated STAT6 could affect cellular gene expression directly. Previously, unphosphorylated STAT proteins (U-STATs) have been shown to influence gene transcription through mechanisms distinct from those used by phosphorylated STAT (Yang et al., 2007; Yang & Stark, 2008). For example, U-STAT1 mediates constitutive expression of the low-molecular-mass polypeptide (LMP) 2 gene by collaborating with IRF1 (Chatterjee-Kishore et al., 1998). U-STAT3 also binds to unphosphorylated NF-κB in competition with IκB, and the resulting U-STAT3–NF-κB complex accumulates in the nucleus with help from the nuclear-localization signal of STAT3, eventually activating a subset of NF-κB-dependent genes (Yang et al., 2007). Moreover, U-STAT6 cooperates with p300 and binds to a consensus STAT6-binding site located within the COX-2 promoter to enhance COX-2 expression in human non-small-cell lung cancer (Cui et al., 2007). Constitutively expressed COX-2 produces a high level of PGE2, which increases resistance to apoptosis, promotes angiogenesis and suppresses anti-tumour immunity (Sheng et al., 1998; Stolina et al., 2000). Second, U-STATs may be associated with maintaining the stability of transcriptionally repressed heterochromatin and controlling cellular epigenetic status, which affects expression of genes beyond those under direct STAT transcription control (Li, 2008). It was shown that U-STAT (U-STAT92E) of Drosophila melanogaster interacts physically with heterochromatin protein 1 (HP1) to promote heterochromatin stability and that the unphosphorylated or ‘transcriptionally inactive’ form of STAT92E is required for stabilizing HP1 localization and histone H3 Lys9 methylation (H3mK9) (Shi et al., 2008). Increased levels of heterochromatin caused by U-STAT in Drosophila resulted in diminished DNA damage and increased survival rate under genotoxic stress such as irradiation (Yan et al., 2011). The changes in chromatin structure associated with constitutive STAT activation or the level of U-STATs in the nucleus often found in cancer cells might be associated with their ability to regenerate a tumour (Brown & Zeidler, 2008). However, when monitored using confocal microscopy, U-STAT6 in T-cells expressing Tip did not colocalize with HP1 protein in the nucleus (data not shown), suggesting that the nuclear U-STAT6 induced by Tip may not be involved in the control of heterochromatin stability.
Recently, Cai et al. (2010) demonstrated that Kaposi’s sarcoma-associated herpesvirus (KSHV) suppresses IL-4-induced signalling by reducing the phosphorylation of STAT6 and its DNA-binding affinity. They showed that KSHV-encoded LANA is essential for viral blocking of IL-4-mediated STAT6 activation. However, it was also observed that knockdown of endogenous STAT6 dramatically increases the sensitivity of KSHV-positive primary effusion lymphoma cells to low-serum stress or chemically mediated cellular apoptosis, and the basal level of constitutive phosphorylation of STAT6 in KSHV-positive cell lines (Cai et al., 2010). In addition, which viral gene is responsible for the constitutive activation of STAT6 in KSHV-infected cells remains to be elucidated. As an oncogenic γ2 herpesvirus homologue of KSHV, we now show that HVS is equipped with a viral protein, Tip, that can activate the STAT6 transcription factor constitutively in Jurkat T-cells and may ultimately contribute to IL-2-independent T-cell transformation. The functional activation and role of STAT6 in T-cell transformation need to be verified further in primary T-cells and HVS-transformed human T-cells in the future.
Methods
Cell culture and reagents.
Jurkat T-cells (clone E6-1; ATCC TIB-152) and 293T cells were grown in RPMI 1640 and Dulbecco’s modified Eagle’s medium, respectively, both supplemented with 10 % FBS and 1 % penicillin/streptomycin (Gibco-BRL, Invitrogen). Jurkat T-cells were electroporated using a Bio-Rad electroporator at 260 V and 975 µF in RPMI 1640 medium in the absence of antibiotics. 293T cells were transiently transfected using calcium phosphate (Clontech). All chemicals were purchased from Sigma-Aldrich unless otherwise noted. Antibodies used were as follows: anti-Tip antibody was generated in a rabbit as described previously (Biesinger et al., 1995). Anti-Lck and anti-GFP antibodies were obtained from Santa Cruz Biotechnology and anti-STAT3, anti-phosphoSTAT3 (Y705), anti-STAT6 and anti-phosphoSTAT6 (Y641) antibodies were purchased from Cell Signaling Technology. For confocal microscopy analysis, anti-STAT6 antibody (BD Transduction Laboratories) was used. Anti-mouse or -rabbit antibodies conjugated to horseradish peroxidase (Santa Cruz Biotechnology) or to Alexa 488 or Alexa 594 (Molecular Probes, Invitrogen) were used for immunoblotting and confocal microscopy, respectively. GFP fusions of Tip or its mutants were made using pEGFP-C2 plasmids (Clontech). The sequences changed in each mutant were as follows: TipmLBD, 150-Ser/Phe/Leu-152 to Ala/Ala/Ala, and prolines at aa 175, 177, 178, 180 and 181 to Ala (Cho et al., 2004, 2006); TipΔTM, deletion from aa 227-Ile to 256-Ser (Cho et al., 2006); TipΔ2, deletion from aa 44-His to 96-Ser (Park et al., 2003); TipAmp1, 216-Ile, 220-Leu, 223-Leu and 227-Ile to Lys (Min et al., 2008).
GST pull-down assay and protein identification by mass spectrometry.
GST-fusion proteins were purified from either E. coli strain BL21(DE3) or TKX1, which contains a mammalian elk tyrosine kinase expression vector (Stratagene). Jurkat T-cell lysates were incubated with glutathione beads containing GST-fusion protein in binding buffer [20 mM HEPES (pH 7.4), 100 mM NaCl, 1 % NP-40, protease inhibitors] at 4 °C for 2 h. Glutathione beads were then washed four times with binding buffer, and the proteins associated with the beads were analysed by SDS-PAGE (Lee et al., 2005). The purified proteins were visualized by silver staining (Invitrogen) and unique protein bands were cut out and sent to the Taplin Biological Mass Spectrometry facility at the Harvard Medical School (Boston, MA, USA) for mass spectrometry analysis.
Immunoprecipitation and immunoblotting.
Cell lysates were prepared as above in 0.5 % NP-40 or RIPA buffer [50 mM Tris/HCl (pH 7.5), 150 mM NaCl, 1 % NP-40, 0.5 % sodium deoxycholate, 0.1 % SDS] and pre-cleared with protein A/G beads for 2 h before immunoprecipitation. Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to PVDF membranes (Millipore), blocked in 5 % milk in Tris-buffered saline with 0.05 % Tween 20, and incubated with primary antibodies, followed by secondary antibodies conjugated to horseradish peroxidase. Immunoblot detection of proteins was performed by using an enhanced chemiluminescence system (Pierce).
Confocal microscopy.
Cells were fixed with 4 % paraformaldehyde for 15 min and permeabilized with 0.2 % Triton X-100 for 15 min. After blocking with 1 % BSA in PBS, the cells were reacted with the appropriate primary antibody at 4 °C overnight. Alexa 488- or Alexa 594-conjugated anti-rabbit or anti-mouse antibodies (Molecular Probes) were used as secondary antibodies. Nuclei were stained with TO-PRO-3 iodide (Molecular Probes) at room temperature for 15 min. Confocal microscopy was performed using an Olympus FV1000 laser-scanning microscope (Olympus) with a ×60 Olympus objective. Images were collected at 512×512 pixel resolution using Olympus imaging software. The stained cells were sectioned optically in the z-axis, and the images in the different channels (photomultiplier tubes) were collected sequentially. The images were rendered using Olympus Fluoview v1.6b or Adobe Photoshop software.
Luciferase reporter assays.
Jurkat cells were electroporated with plasmids encoding STAT6 and Tip or its mutant together with the STAT6-responsive luciferase reporter plasmid (3×STAT6-luc: pTransLucent containing three tandem repeats of pSTAT6-binding sites) provided by Karen Leroy (Université Paris, France) (Ritz et al., 2009). To normalize transfection efficiency, the pGK-βgal vector, which expresses β-galactosidase from a phosphoglucokinase promoter, was included in the transfection mixture. Cells were then sampled at 24 h post-electroporation. For IL-4 (Cell Signaling Technology) treatment, cells were incubated with 20 ng IL-4 ml−1 for 18 h prior to cell lysis. Cells were harvested and washed once in cold PBS. The cells were then lysed in 100 µl lysis buffer [0.1 mM potassium phosphate buffer (pH 7.8), 1 % Triton X-100, 1 mM DTT and 2 mM EDTA]. Cells were incubated in lysis buffer for 15–20 min on ice before pelleting in a microcentrifuge for 2 min at high speed (20 000 g) to remove cellular debris. Cell lysate (30 µl) was mixed with 100 µl assay buffer (30 mM Tricine, 3 mM ATP, 15 mM MgSO4, 10 mM DTT, pH 7.8) in a 96-well microtitre plate just prior to measurement. Plates were assayed using the MLX microtitre luminescence detection system (Dynex Technologies), which injects 100 µl of the substrate (1 mM d-luciferin in assay buffer) prior to measuring luminescence. Assays were performed in triplicate.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MEST) (2009-0070600 and 2010-0019472).
References
- Benekli M., Baer M. R., Baumann H., Wetzler M. (2003). Signal transducer and activator of transcription proteins in leukemias. Blood 101, 2940–2954 10.1182/blood-2002-04-1204 [DOI] [PubMed] [Google Scholar]
- Biesinger B., Müller-Fleckenstein I., Simmer B., Lang G., Wittmann S., Platzer E., Desrosiers R. C., Fleckenstein B. (1992). Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci U S A 89, 3116–3119 10.1073/pnas.89.7.3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesinger B., Tsygankov A. Y., Fickenscher H., Emmrich F., Fleckenstein B., Bolen J. B., Bröker B. M. (1995). The product of the Herpesvirus saimiri open reading frame 1 (Tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem 270, 4729–4734 10.1074/jbc.270.9.4729 [DOI] [PubMed] [Google Scholar]
- Bild A. H., Turkson J., Jove R. (2002). Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J 21, 3255–3263 10.1093/emboj/cdf351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T., Garcia R., Turkson J., Jove R. (2000). STATs in oncogenesis. Oncogene 19, 2474–2488 10.1038/sj.onc.1203527 [DOI] [PubMed] [Google Scholar]
- Bromberg J., Darnell J. E., Jr (2000). The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19, 2468–2473 10.1038/sj.onc.1203476 [DOI] [PubMed] [Google Scholar]
- Brown S., Zeidler M. P. (2008). Unphosphorylated STATs go nuclear. Curr Opin Genet Dev 18, 455–460 10.1016/j.gde.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Cai Q., Verma S. C., Choi J. Y., Ma M., Robertson E. S. (2010). Kaposi’s sarcoma-associated herpesvirus inhibits interleukin-4-mediated STAT6 phosphorylation to regulate apoptosis and maintain latency. J Virol 84, 11134–11144 10.1128/JVI.01293-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee-Kishore M., Kishore R., Hicklin D. J., Marincola F. M., Ferrone S. (1998). Different requirements for signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 in the regulation of low molecular mass polypeptide 2 and transporter associated with antigen processing 1 gene expression. J Biol Chem 273, 16177–16183 10.1074/jbc.273.26.16177 [DOI] [PubMed] [Google Scholar]
- Cho N. H., Feng P., Lee S. H., Lee B. S., Liang X., Chang H., Jung J. U. (2004). Inhibition of T cell receptor signal transduction by tyrosine kinase-interacting protein of Herpesvirus saimiri. J Exp Med 200, 681–687 10.1084/jem.20040924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N. H., Kingston D., Chang H., Kwon E. K., Kim J. M., Lee J. H., Chu H., Choi M. S., Kim I. S., Jung J. U. (2006). Association of herpesvirus saimiri Tip with lipid raft is essential for downregulation of T-cell receptor and CD4 coreceptor. J Virol 80, 108–118 10.1128/JVI.80.1.108-118.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. K., Ishido S., Jung J. U. (2000). The collagen repeat sequence is a determinant of the degree of herpesvirus saimiri STP transforming activity. J Virol 74, 8102–8110 10.1128/JVI.74.17.8102-8110.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Zhang L., Luo J., Rajasekaran A., Hazra S., Cacalano N., Dubinett S. M. (2007). Unphosphorylated STAT6 contributes to constitutive cyclooxygenase-2 expression in human non-small cell lung cancer. Oncogene 26, 4253–4260 10.1038/sj.onc.1210222 [DOI] [PubMed] [Google Scholar]
- Deutsch H. H. J., Koettnitz K., Chung J., Kalthoff F. S. (1995). Distinct sequence motifs within the cytoplasmic domain of the human IL-4 receptor differentially regulate apoptosis inhibition and cell growth. J Immunol 154, 3696–3703 [PubMed] [Google Scholar]
- Duboise S. M., Guo J., Czajak S., Desrosiers R. C., Jung J. U. (1998). STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol 72, 1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensser A., Thurau M., Wittmann S., Fickenscher H. (2003). The genome of herpesvirus saimiri C488 which is capable of transforming human T cells. Virology 314, 471–487 10.1016/S0042-6822(03)00449-5 [DOI] [PubMed] [Google Scholar]
- Guiter C., Dusanter-Fourt I., Copie-Bergman C., Boulland M. L., Le Gouvello S., Gaulard P., Leroy K., Castellano F. (2004). Constitutive STAT6 activation in primary mediastinal large B-cell lymphoma. Blood 104, 543–549 10.1182/blood-2003-10-3545 [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Cooper G. M. (2000). Direct binding and activation of STAT transcription factors by the herpesvirus saimiri protein Tip. J Biol Chem 275, 16925–16932 10.1074/jbc.M000709200 [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Amdjadi K., Hurley T. R., Lund T. C., Medveczky P. G., Sefton B. M. (2000). Activation of the Lck tyrosine protein kinase by the herpesvirus saimiri tip protein involves two binding interactions. Virology 276, 339–348 10.1006/viro.2000.0570 [DOI] [PubMed] [Google Scholar]
- Haura E. B., Turkson J., Jove R. (2005). Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2, 315–324 10.1038/ncponc0195 [DOI] [PubMed] [Google Scholar]
- Heck E., Lengenfelder D., Schmidt M., Müller-Fleckenstein I., Fleckenstein B., Biesinger B., Ensser A. (2005). T-cell growth transformation by herpesvirus saimiri is independent of STAT3 activation. J Virol 79, 5713–5720 10.1128/JVI.79.9.5713-5720.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck E., Friedrich U., Gack M. U., Lengenfelder D., Schmidt M., Müller-Fleckenstein I., Fleckenstein B., Ensser A., Biesinger B. (2006). Growth transformation of human T cells by herpesvirus saimiri requires multiple Tip–Lck interaction motifs. J Virol 80, 9934–9942 10.1128/JVI.01112-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. (1994). An interleukin-4-induced transcription factor: IL-4 Stat. Science 265, 1701–1706 10.1126/science.8085155 [DOI] [PubMed] [Google Scholar]
- Ilaria R. L., Jr, Van Etten R. A. (1996). P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem 271, 31704–31710 10.1074/jbc.271.49.31704 [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O’Shea J. J. (1994). Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370, 151–153 10.1038/370151a0 [DOI] [PubMed] [Google Scholar]
- Jung J. U., Lang S. M., Friedrich U., Jun T., Roberts T. M., Desrosiers R. C., Biesinger B. (1995). Identification of Lck-binding elements in Tip of herpesvirus saimiri. J Biol Chem 270, 20660–20667 10.1074/jbc.270.35.20660 [DOI] [PubMed] [Google Scholar]
- Jung J. U., Choi J. K., Ensser A., Biesinger B. (1999). Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin Cancer Biol 9, 231–239 10.1006/scbi.1998.0115 [DOI] [PubMed] [Google Scholar]
- Keegan A. D., Nelms K., White M., Wang L. M., Pierce J. H., Paul W. E. (1994). An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell 76, 811–820 10.1016/0092-8674(94)90356-5 [DOI] [PubMed] [Google Scholar]
- Kjellen P., Amdjadi K., Lund T. C., Medveczky P. G., Sefton B. M. (2002). The herpesvirus saimiri Tip484 and Tip488 proteins both stimulate lck tyrosine protein kinase activity in vivo and in vitro. Virology 297, 281–288 10.1006/viro.2002.1419 [DOI] [PubMed] [Google Scholar]
- Lee H., Choi J. K., Li M., Kaye K., Kieff E., Jung J. U. (1999). Role of cellular tumor necrosis factor receptor-associated factors in NF-κB activation and lymphocyte transformation by herpesvirus saimiri STP. J Virol 73, 3913–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. S., Lee S. H., Feng P., Chang H., Cho N. H., Jung J. U. (2005). Characterization of the Kaposi’s sarcoma-associated herpesvirus K1 signalosome. J Virol 79, 12173–12184 10.1128/JVI.79.19.12173-12184.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Darnell J. E., Jr (2002). Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3, 651–662 10.1038/nrm909 [DOI] [PubMed] [Google Scholar]
- Li W. X. (2008). Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol 18, 545–551 10.1016/j.tcb.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T. C., Garcia R., Medveczky M. M., Jove R., Medveczky P. G. (1997). Activation of STAT transcription factors by herpesvirus saimiri Tip-484 requires p56lck. J Virol 71, 6677–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T. C., Prator P. C., Medveczky M. M., Medveczky P. G. (1999). The Lck binding domain of herpesvirus saimiri Tip-484 constitutively activates Lck and STAT3 in T cells. J Virol 73, 1689–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min C. K., Bang S. Y., Cho B. A., Choi Y. H., Yang J. S., Lee S. H., Seong S. Y., Kim K. W., Kim S. & other authors (2008). Role of amphipathic helix of a herpesviral protein in membrane deformation and T cell receptor downregulation. PLoS Pathog 4, e1000209 10.1371/journal.ppat.1000209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Noguchi P. D., Puri R. K. (1996). IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol 156, 2972–2978 [PubMed] [Google Scholar]
- Murphy C., Kretschmer C., Biesinger B., Beckers J., Jung J., Desrosiers R. C., Müller-Hermelink H. K., Fleckenstein B. W., Rüther U. (1994). Epithelial tumours induced by a herpesvirus oncogene in transgenic mice. Oncogene 9, 221–226 [PubMed] [Google Scholar]
- Park J., Lee B. S., Choi J. K., Means R. E., Choe J., Jung J. U. (2002). Herpesviral protein targets a cellular WD repeat endosomal protein to downregulate T lymphocyte receptor expression. Immunity 17, 221–233 10.1016/S1074-7613(02)00368-0 [DOI] [PubMed] [Google Scholar]
- Park J., Cho N. H., Choi J. K., Feng P., Choe J., Jung J. U. (2003). Distinct roles of cellular Lck and p80 proteins in herpesvirus saimiri Tip function on lipid rafts. J Virol 77, 9041–9051 10.1128/JVI.77.16.9041-9051.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. Z., Kamarashev J., Zhang C. L., Dummer R., Burg G., Döbbeling U. (2001). Constitutive and interleukin-7- and interleukin-15-stimulated DNA binding of STAT and novel factors in cutaneous T cell lymphoma cells. J Invest Dermatol 117, 583–589 10.1046/j.0022-202x.2001.01436.x [DOI] [PubMed] [Google Scholar]
- Reichel M., Nelson B. H., Greenberg P. D., Rothman P. B. (1997). The IL-4 receptor alpha-chain cytoplasmic domain is sufficient for activation of JAK-1 and STAT6 and the induction of IL-4-specific gene expression. J Immunol 158, 5860–5867 [PubMed] [Google Scholar]
- Ritz O., Guiter C., Castellano F., Dorsch K., Melzner J., Jais J. P., Dubois G., Gaulard P., Möller P., Leroy K. (2009). Recurrent mutations of the STAT6 DNA binding domain in primary mediastinal B-cell lymphoma. Blood 114, 1236–1242 10.1182/blood-2009-03-209759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., McReynolds L. J., Keegan A., Wang L. H., Garfein E., Rothman P., Nelms K., Paul W. E. (1996). Growth and gene expression are predominantly controlled by distinct regions of the human IL-4 receptor. Immunity 4, 123–132 10.1016/S1074-7613(00)80677-9 [DOI] [PubMed] [Google Scholar]
- Shao H., Xu X., Mastrangelo M. A., Jing N., Cook R. G., Legge G. B., Tweardy D. J. (2004). Structural requirements for signal transducer and activator of transcription 3 binding to phosphotyrosine ligands containing the YXXQ motif. J Biol Chem 279, 18967–18973 10.1074/jbc.M314037200 [DOI] [PubMed] [Google Scholar]
- Sheng H., Shao J., Morrow J. D., Beauchamp R. D., DuBois R. N. (1998). Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 58, 362–366 [PubMed] [Google Scholar]
- Shi S., Larson K., Guo D., Lim S. J., Dutta P., Yan S. J., Li W. X. (2008). Drosophila STAT is required for directly maintaining HP1 localization and heterochromatin stability. Nat Cell Biol 10, 489–496 10.1038/ncb1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinnider B. F., Elia A. J., Gascoyne R. D., Patterson B., Trumper L., Kapp U., Mak T. W. (2002). Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed–Sternberg cells of Hodgkin lymphoma. Blood 99, 618–626 10.1182/blood.V99.2.618 [DOI] [PubMed] [Google Scholar]
- Stolina M., Sharma S., Lin Y., Dohadwala M., Gardner B., Luo J., Zhu L., Kronenberg M., Miller P. W. & other authors (2000). Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol 164, 361–370 [DOI] [PubMed] [Google Scholar]
- Wehner L. E., Schröder N., Kamino K., Friedrich U., Biesinger B., Rüther U. (2001). Herpesvirus saimiri Tip gene causes T-cell lymphomas in transgenic mice. DNA Cell Biol 20, 81–88 10.1089/104454901750070283 [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. (1994). Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature 370, 153–157 10.1038/370153a0 [DOI] [PubMed] [Google Scholar]
- Xu F., Mukhopadhyay S., Sehgal P. B. (2007). Live cell imaging of interleukin-6-induced targeting of ‘transcription factor’ STAT3 to sequestering endosomes in the cytoplasm. Am J Physiol Cell Physiol 293, C1374–C1382 10.1152/ajpcell.00220.2007 [DOI] [PubMed] [Google Scholar]
- Yan S. J., Lim S. J., Shi S., Dutta P., Li W. X. (2011). Unphosphorylated STAT and heterochromatin protect genome stability. FASEB J 25, 232–241 10.1096/fj.10-169367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Stark G. R. (2008). Roles of unphosphorylated STATs in signaling. Cell Res 18, 443–451 10.1038/cr.2008.41 [DOI] [PubMed] [Google Scholar]
- Yang J., Liao X., Agarwal M. K., Barnes L., Auron P. E., Stark G. R. (2007). Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFκB. Genes Dev 21, 1396–1408 10.1101/gad.1553707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Pardoll D., Jove R. (2009). STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9, 798–809 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S. & other authors (1993). SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778 10.1016/0092-8674(93)90404-E [DOI] [PubMed] [Google Scholar]