Abstract
Salmon life histories are finely tuned to local environmental conditions, which are intimately linked to climate. We summarize the likely impacts of climate change on the physical environment of salmon in the Pacific Northwest and discuss the potential evolutionary consequences of these changes, with particular reference to Columbia River Basin spring/summer Chinook (Oncorhynchus tshawytscha) and sockeye (Oncorhynchus nerka) salmon. We discuss the possible evolutionary responses in migration and spawning date egg and juvenile growth and development rates, thermal tolerance, and disease resistance. We know little about ocean migration pathways, so cannot confidently suggest the potential changes in this life stage. Climate change might produce conflicting selection pressures in different life stages, which will interact with plastic (i.e. nongenetic) changes in various ways. To clarify these interactions, we present a conceptual model of how changing environmental conditions shift phenotypic optima and, through plastic responses, phenotype distributions, affecting the force of selection. Our predictions are tentative because we lack data on the strength of selection, heritability, and ecological and genetic linkages among many of the traits discussed here. Despite the challenges involved in experimental manipulation of species with complex life histories, such research is essential for full appreciation of the biological effects of climate change.
Keywords: genetic correlation, global warming, phenological change, smolt timing
Introduction
Climate change is transforming the fitness landscapes of millions of species at a rapid rate, but we have little understanding of the evolutionary consequences. Evolutionary responses to climate change are important because nongenetic responses, such as shifts of range edges and plastic phenotypic change, might not be sufficient for the persistence of many populations (Sala et al. 2000; Thomas et al. 2004), and because strong selection increases the risk of extinction in small populations (Bürger and Lynch 1995). Evolutionary responses seem likely because of the prevalence of spatial variation in physiological and behavioral traits that reflect past adaptation to local climate (Garland and Adolph 1991; Davis et al. 2005; Reusch and Wood 2007), and growing evidence of contemporary evolution in response to a variety of environmental disturbances (Stockwell et al. 2003). However, despite a few notable exceptions, genetic responses to climate change have proven difficult to demonstrate (see reviews in Bradshaw and Holzapfel 2006; Reusch and Wood 2007; Gienapp et al. 2008). Most phenotypic change recently observed might be largely due to plastic (i.e. nongenetic) change (Reale et al. 2003a; Berteaux et al. 2004; Gienapp et al. 2008). Persistence through climate change will continue to depend on plastic responses, because evolutionary responses are often limited and can impose demographic costs (Lynch and Lande 1993; Bürger and Lynch 1995). Furthermore, the distinction between genetic and plastic responses is simplistic because populations show genetic differences in their plasticity, and these ‘norms of reaction’ can evolve (Nussey et al. 2005). Plastic and genetic mechanisms interact in complicated ways, and it is important to disentangle them in order to predict the effects of climate change on natural populations.
Most empirical research to date has considered evolutionary responses to environmental change in single traits, such as a shift in photoperiodic cues for diapause in mosquitoes (Bradshaw and Holzapfel 2006), dispersal ability in crickets (Thomas et al. 2001) and butterflies (Hill et al. 1999), or chromosome inversion rates in Drosophila (Balanya et al. 2006). This single-trait approach yields insights, but selection is often more complicated. For example, in species with complex life histories, selection due to climate change can act simultaneously on multiple traits in ways that differ through the life cycle (Prout 1971; Lande and Arnold 1983; Arnold and Wade 1984; Lynch 1999). Changes in one life stage can have extensive repercussions for later life stages, particularly in migratory animals, where multiple life-stage transitions are finely tuned to conditions in radically different environments. Genetic covariances between traits under different selection pressures will shape the response to selection (Etterson and Shaw 2001). Moreover, community interactions are likely to be disturbed, simply because phenological responses of interacting species might not be parallel (Harrington et al. 1999; Visser and Both 2005).
We explore how these various mechanisms might interact to shape the selective environment in the case of Pacific salmon (Oncorhynchus spp.). Salmon species have plastic life histories, but adaptation of reaction norms to local environmental conditions at a very fine spatial scale (e.g. Tallman 1986; Quinn et al. 2000; Beer and Anderson 2001; Keefer et al. 2004) suggests that climate change will profoundly affect salmon life histories, and the interplay between genetic and plastic responses is likely to be important. The anadromous salmon life cycle depends crucially on appropriate timing of transitions between habitats, so the potential for a growing mismatch between the needs of different stages in relation to these transitions is a major concern. Many salmon populations in the Pacific Northwest are already threatened with extinction, so the effects of climate on absolute fitness (i.e. a population’s capacity for replacing itself) warrant conservation concern, and must be considered in the context of a web of natural and anthropogenic agents of selection (Waples et al. 2008).
A consequence of extensive local adaptation and life-history diversity in salmon is that climate change will differ in its effects on specific populations; review of all these effects is beyond the scope of this paper. Rather, we emphasize the interacting and cumulative effects of climate change across the life cycle. To accomplish this, we focus on a particular set of Chinook (Oncorhynchus tshawytscha) and sockeye (Oncorhynchus nerka) populations having certain life-history commonalities (namely spring adult migration and yearling juvenile outmigration) within the Columbia River Basin. The Columbia River marks the southern limit of the geographic range of sockeye, but it is well within the range of Chinook salmon (Groot and Margolis 1991). Chinook salmon persist south of the Columbia River in Oregon and California and were abundant historically, but these populations are genetically and behaviorally very distinct from the populations considered here. Snake River spring/summer Chinook salmon that are our primary focus are the southernmost populations of a northern ecotype of Chinook, defined by a combination of juvenile seaward migration timing, ocean migration pattern, and the season of adult return (Taylor 1990; Healey 1991; Waples et al. 2004). We argue here that these characteristics will become increasingly maladapted with climate change. These populations are listed as threatened under the US Endangered Species Act (NMFS 1992), so any further decline in fitness significantly threatens their persistence (McClure et al. 2003; Crozier et al. 2008).
In the following sections, we first explain the complex nature of Pacific salmon life histories and their adaptations to diverse environments across the Pacific Rim. We then consider how these environments, particularly those experienced by our focal populations, are expected to change due to climate warming. We next examine evidence for local adaptation to climate, likely changes in selection with climate change, and potential evolutionary responses for certain traits during particular life stages. Finally, we discuss the importance of integrating potential plastic and evolutionary responses across multiple traits and life-history stages.
Salmon life-history diversity
Pacific salmon have complex life histories that span diverse environments across the Pacific Rim (Groot and Margolis 1991; Quinn 2005). They spawn in fall in fresh water and their embryos incubate in the gravel during the winter and emerge in spring. Juveniles then spend days to years in habitats ranging from small creeks to large rivers, and small ponds to large lakes. Most juveniles then migrate downriver, through estuaries and coastal waters, to the ocean. These ‘anadromous’ individuals spend anywhere from a few months to as much as 7 years at sea, before migrating back to spawn and die at their natal sites in fresh water. This great diversity of environments and behaviors suggests that climate change could influence selection on multiple traits in multiple phases of the life cycle.
Life-history diversity in salmon reflects a combination of phenotypic plasticity in response to variable environmental conditions (Hutchings 2004) and local adaptation throughout the life cycle, across the complete suite of life history, morphological, physiological, and behavioral traits (Ricker 1972; Groot and Margolis 1991; Taylor 1991; Quinn 2005). Phenotypic plasticity facilitates rapid colonization of new habitats and immediate responses to environmental change (Quinn et al. 2001; Price et al. 2003; Ghalambor et al. 2007). Local adaptation is facilitated by strong natal homing that limits gene flow between populations in different selective environments. Despite the remarkable extent of plasticity and local adaptation, appropriate and sufficient responses to climate change are not assured because of the uncertain rate and nature of climate change, the genetic properties of traits, the effects of invasive species, and other stressors (e.g. hatcheries, fishing, hydroelectric dams).
Expected climate change
Projections for 21st century climate around the Pacific Rim and in the Pacific Ocean suggest significant surface warming trends, especially at higher latitudes and over continents. A range of models and greenhouse gas and aerosol emissions scenarios project global average warming from ∼+1 to +6°C by the year 2100 (IPCC 2007). For the Pacific Northwest (coastal North America from northern California to southern British Columbia, Fig. 1), warming is projected to be near the global average. Most climate models project modest increases in winter precipitation for this region (on average, ∼10%), but projections for summer precipitation form no consistent pattern (Salathé 2006).
Figure 1.
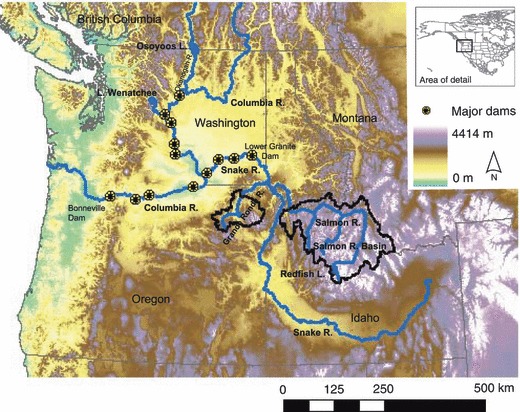
Snake River spring/summer Chinook salmon rear in the Salmon River and Grande Ronde River Basins. Most Columbia River Sockeye rear in Lake Wenatchee and Osoyoos Lake, but a few inhabit in Redfish Lake.
Figure 2.
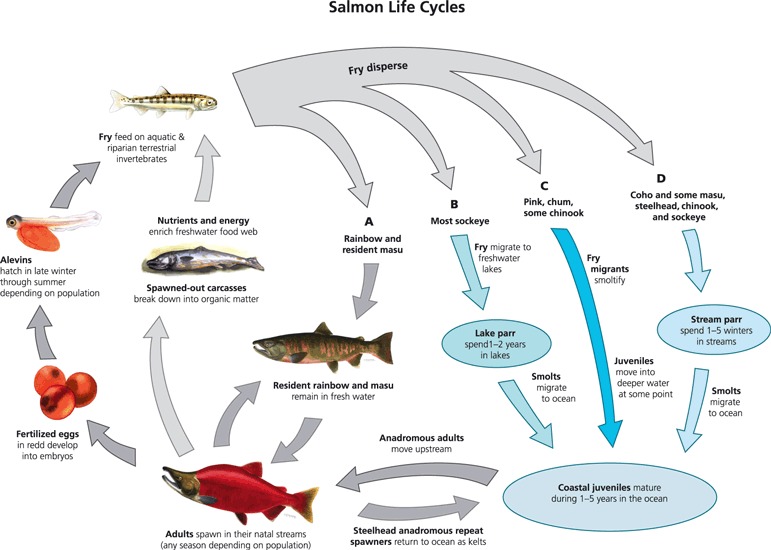
Life cycles for seven genera of Pacific salmonids, illustrating the variety and complexity of anadromous life cycles in salmon, from Augerot (2005).
These climate-change projections indicate clear hydrologic changes for salmon-bearing streams in western North America. Winter will become milder, causing more precipitation to fall as rain and less as snow in locations where surface temperatures have historically been near freezing. A warming climate in the second half of the 20th century caused a significant advance in timing of snowmelt runoff for many rivers in the region (Stewart et al. 2005). Additional warming is expected to cause further shifts in the onset of snowmelt in streams that now carry a substantial snowpack into the spring and summer seasons. A warmer atmosphere has a higher capacity for water vapor, which promotes greater hydrologic extremes: more severe drought in summer and more intense precipitation and flooding in winter. Rising surface air temperatures will also cause stream and estuary temperatures to rise. Over the North Pacific Ocean, important changes in salmon habitat will depend primarily on (i) rising upper ocean temperatures that increase the stratification of the upper ocean, (ii) changes in surface wind patterns, potentially changing the timing and intensity of the upwelling of nutrient-rich subsurface water, and (iii) increasing ocean acidification changing plankton community composition with effects cascading through marine food webs.
This is the template of climate change that is expected to influence the evolution of Pacific salmon in the 21st century. We now explore the evolutionary implications of these trends for the phenology of critical periods in the life history of salmon. For each trait, we (i) describe how climate change might alter the selective regime, (ii) review the trait’s genetic variation and heritability (h2), and (iii) assess the likelihood and relative speed of potential evolutionary responses. It is important to remember that the following conclusions are merely hypotheses, in part because few studies have formally measured selection on salmon in response to environmental change. We do cite those studies but more are certainly needed.
Potential evolutionary pressures and responses
Heat tolerance
The most obvious effect of climate change will be higher temperatures in fresh water. Warmer water can accelerate growth and development where temperatures are below optimal, or stress fish if they cannot behaviorally avoid temperatures that are above optimal. Fitness in warm water is reduced by mortality at lethal temperatures, and various impacts at sublethal temperatures, such as increased susceptibility to warm-water diseases, inhibition of normal behavior, growth and development, especially smoltification, maturation, and egg development, and increased energetic costs (for reviews, see McCullough 1999; Materna 2001). Despite the high elevation at which most of the populations considered here spawn and rear, much of the rearing habitat already exceeds optimal temperatures for salmonids at times (Donato 2002). Temperatures approach lethal limits in the mainstem Columbia, Snake, and Okanagan Rivers regularly, affecting the times fish can migrate to and from the ocean (Hodgson and Quinn 2002; Hyatt et al. 2003; Brannon et al. 2004; Naughton et al. 2005).
Variation in temperature-specific survival rates occurs among populations from different thermal regimes, suggesting that thermal tolerance can evolve in the wild. For example, coastal Chinook salmon populations show lower egg and embryo survival and lower yolk conversion efficiency at cold temperatures than do interior populations (Beacham and Murray 1989), and juvenile Chinook salmon from southern British Columbia tolerate longer exposure to high temperatures than those from northern British Columbia (Beacham and Withler 1991). Beacham and Withler (1991) found heritability for heat tolerance to be significant in the population from the cooler stream (h2 = 0.27), but not in the population from the warmer stream (h2 = 0.00), suggesting that selection had acted in the latter population to increase heat resistance but that further evolutionary potential is limited. Nonetheless, differences in upper lethal temperatures between populations from very different thermal environments are subtle and sometimes disappear with appropriate acclimation and testing (e.g. Brett 1956; Konecki et al. 1995a,b). Overall, these and other studies suggest a potential for local adaptation of heat tolerance, but the limitation of salmon to habitat below ∼23°C (McCullough 1999) points to an ultimate upper limit to heat tolerance that evolution cannot surmount.
Populations near this upper thermal limit seem to persist through behaviors that reduce exposure to the highest temperatures, such as the occupation of cold-water refugia (Berman and Quinn 1991; Torgersen et al. 1999; Goniea et al. 2006). From the perspective of climate-induced warming, it would be valuable to know whether populations differ genetically in their tendency to adopt these behaviors. If all populations harbor the potential for behavioral avoidance of warm water, then these responses might ameliorate some of the effects of climate change except in sites lacking thermal refuges. If not, use of such refuges might depend on the evolution of appropriate behaviors, and the potential for this is entirely unknown.
Disease resistance
Many parasitic and bacterial diseases infect salmon, and some of these infections become more virulent with increasing temperature (McCullough 1999). Reasons for this include lower host resistance when the fish are thermally stressed, and higher pathogen population growth rates, due to shorter generation times at higher temperatures (Marcogliese 2001). Diseases of wild salmon likely to become a greater problem with warmer temperatures include those caused by the myxosporidian parasite Ceratomyxa shasta, the bacterium Flexibacter columnaris, and by various Aeromonas and Listonella species (McCullough 1999). These pathogens are ubiquitous and infection rates can be very high (Ordal and Pacha 1963; Chapman 1986; Tiffan et al. 1996). As the availability of cool water decreases, mortality rates will likely increase and selection should favor increased resistance to these diseases.
Salmon populations that have been exposed to particular diseases historically tend to have higher resistance to those diseases (Zinn et al. 1977; Bower et al. 1995; Bartholomew 1998; Miller and Vincent 2008). The Columbia River has already undergone changes (increased temperature, lower flows, slower juvenile migration) that probably have increased exposure and susceptibility to certain diseases. Ordal and Pacha (1963) identified high columnaris infection rates as a potential cause for the decline of Columbia River Chinook, sockeye, and steelhead trout in the early 1960s. Although experiments are complicated by enormous variability in strain virulence, it would be informative to see if resistance has increased compared with their findings, and those of Zinn et al. (1977).
The rate at which resistance responds to changes in pathogen prevalence or virulence will depend in part on its heritability. Heritabilities for resistance to common diseases range from very low to moderate, but tend to be lower in populations that have historically been exposed to the disease (0–0.34, Beacham and Evelyn 1992; 0.13, Hard et al. 2006). Low heritabilities will limit the pace of future adaptation in populations that already show some resistance, such as our focal populations.
Upstream migration
Snake River spring/summer Chinook salmon spawn in the Grande Ronde River Basin in Oregon and in the Salmon River Basin in central Idaho, at the highest elevations of any salmon population (up to 2000 m above sea level, Fig. 1). They also complete some of the longest migrations: up to 1500 km from the ocean to their spawning sites. Columbia River sockeye salmon migrate up to 1000 km to spawning grounds in the Wenatchee and Osoyoos lakes. A small population persists in Redfish Lake in the Salmon River Basin. Successful spawning in such populations requires that they (i) stay in the ocean long enough to acquire adequate energy stores, (ii) use energy efficiently during migration, (iii) avoid migration when conditions are especially difficult (e.g. high temperatures, very low flow), and (iv) arrive prior to the appropriate spawning date. Climate change will likely alter the optimal balance between these demands owing to changes in temperature and flow that influence mortality and energy costs (Hinch and Rand 1998; Rand et al. 2006; Young et al. 2006).
Most fish in our focal populations migrate up the Columbia River in April and May, prior to peak temperatures, and then hold in deep, cool pools before moving to spawning grounds. Snake River Chinook salmon spawn in mid- to late-August (Good et al. 2005), and Columbia River sockeye salmon spawn in September and October (Hyatt et al. 2003). Migration prior to peak temperatures is presumably necessary in order to complete their long migration prior to the appropriate time for spawning. Late migrants have high mortality during the migration (Naughton et al. 2005) or experience delays while they seek thermal refugia (High et al. 2006; Salinger and Anderson 2006), probably owing to the warmer water in July and August. Mean July water temperature in the Columbia River has risen steadily from 16.9°C in 1950 to 20.9°C in 2006 (measured at Bonneville Dam, Fig. 3; DART 2007). Not only are peak temperatures warmer, but high temperatures last longer; compared with the late 1930s, stressful temperatures now begin a full month earlier and persist 2–3 weeks later (Quinn and Adams 1996). In short, recent selection against migration during stressful summer temperatures has likely favored earlier migration in spring.
Figure 3.
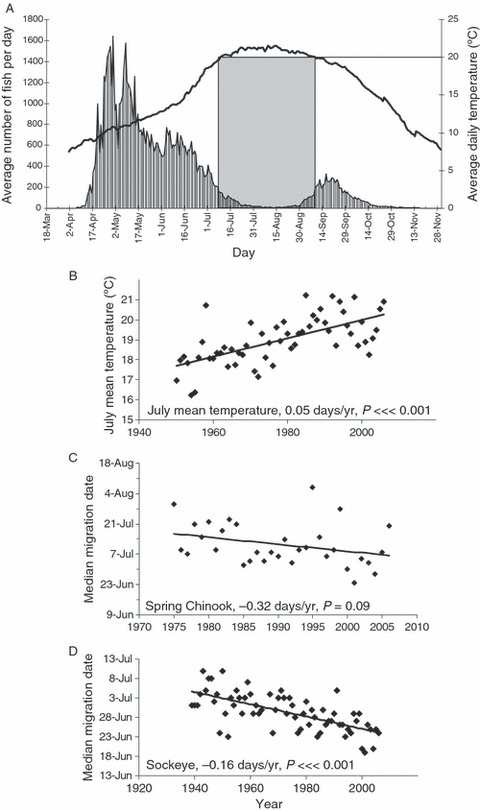
(A) Average daily Chinook salmon counts and temperatures at Lower Granite Dam from 1995 to 2006. The boxed area shows the average time period the river is over 20°C, reducing the migration of adults, as shown by the lower Chinook counts during this time period. (B) Mean July temperature at Bonneville Dam, with 1960–1979 temperatures inferred from measurements at McNary Dam. Median migration date of (C) spring Chinook and (D) sockeye salmon. Regression statistics and lines are shown. All data from DART 2007.
Consistent with this prediction, a trend toward increasingly earlier migration over the past century in spring/summer Chinook and sockeye salmon is evident (Fig. 3, Quinn and Adams 1996). However, the extent to which these responses are plastic or genetic is unclear, and might be confounded with changing abundance of populations that differ in timing, or by changes in hatchery production or harvest. Sea surface temperatures can influence migration timing via plasticity, but this effect tends to be weak (Hodgson and Quinn 2002; Hodgson et al. 2006). Furthermore, the high heritability of timing-related traits (median 0.51, Carlson and Seamons 2008) supports the plausibility of evolutionary adaptation due to strong selection resulting from changing conditions.
Compared with spring Chinook salmon, Columbia River Basin sockeye are more suitable for exploring the probable strength of the selection differential caused by rising temperatures because their simpler population structure and minimum of hatchery propagation simplify the analysis of time trends (Quinn and Adams 1996; Hodgson et al. 2006). Here, earlier migration appears to have evolved owing to warming water conditions in the Columbia River (Quinn and Adams 1996). Recent analyses (L. Crozier, unpublished data) support this conclusion by quantifying thermal selection for earlier migration over the past 50 years. Specifically, sockeye salmon that survive migration are expected to pass Bonneville Dam on average 2.5 days earlier per generation (0.3 SDs) than the population average, based on a probabilistic model of temperature-induced mortality, and historical records of migration time and temperature. With this selection differential, the observed shift in migration timing of 8.6 days (Fig. 3) could be accomplished with a migration-timing heritability of only 0.24. This value is certainly plausible (cf. 1.06, Quinn et al. 2000), indicating that evolutionary change could easily account for the observed trend in migration timing. Even so, the future evolution of migration time will eventually be constrained by eroding genetic variation and conflicting demands. For example, if salmon migrate earlier in the summer but spawn at the same date in fall (or even later), they will need more energy to sustain themselves for the longer period of fasting. This need for more stored energy might be in conflict with the need to leave the ocean earlier in the summer, missing some of the best growing conditions.
Spawning date, emergence date, and development rates
Snake River spring Chinook salmon spawn in the late summer; embryos develop over winter and emerge from the gravel as fry in early spring. In general, emergence timing appears to be under stabilizing selection, because fry have low survival if they emerge too early, before food is seasonally available, or too late to capitalize on crucial growth opportunities (Brannon 1987; Einum and Fleming 2000; Letcher et al. 2004). Embryo development rates are tightly linked to water temperature (Beacham and Murray 1990), so optimal emergence timing must match local conditions through adjustments to spawning date or genetically based, temperature-specific embryo development rates (Brannon 1987; Brannon et al. 2004). Indeed, even small differences in water temperature among nearby spawning locations can influence spawning date (Beer and Anderson 2001). On the other hand, spawning date can sometimes vary for reasons other than selection on emergence timing, such as habitat inaccessibility at a particular time or energetic demands on adults, and in such cases temperature-specific development rates might evolve (Tallman 1986; Tallman and Healey 1991).
Warmer winters will accelerate development and lead to earlier emergence. The optimal time for emergence will also advance, because seasonal initiation of primary and secondary productivity in general is temperature-sensitive. However, fry emergence and optimal food conditions might not advance at the same rate. If emergence date diverges from optimal conditions, then selection should favor compensatory changes in spawning date or temperature-specific development rates. Spawning date is particularly likely to evolve owing to its high heritability in salmonids (Quinn et al. 2000; Hard 2004; Hendry and Day 2005; Carlson and Seamons 2008). In fact, spawning date has evolved quickly in populations transplanted to new environments. Chinook salmon populations transplanted to New Zealand, for example, have diverged several weeks in maturation date, which is closely related to spawning date, in the 80 years since their introduction (Quinn et al. 2000, 2001; Unwin et al. 2000). Moreover, this evolutionary divergence matches expectations: later spawning occurs in the populations where embryos develop in warmer water. Spawning date in Columbia River salmon might thus evolve rapidly in response to climate change, unless artificial propagation of the population exerts countervailing selection (Quinn et al. 2002).
It is less certain whether temperature-specific development rates will evolve with climate warming. Although development rates do seem adapted in some situations to match emergence timing to favorable conditions, the most dramatic variation is among groups that spawn at different times in the same site (Tallman 1986; Brannon 1987; Tallman and Healey 1991; Hendry et al. 1998). Moreover, the heritability of embryo development rate seems much lower (Hebert et al. 1998; Kinnison et al. 1998) than that for spawning date, suggesting that the evolution of development rates will be relatively slow. Consistent with this expectation, the divergence in spawning date among New Zealand Chinook salmon populations was not accompanied by the divergence in temperature-specific development rates (Kinnison et al. 1998; Unwin et al. 2000). However, if changes in spawning date do not lead to optimal emergence timing, changing development rates would be the only evolutionarily mechanism to adjust emergence timing.
Juvenile rearing
After the fry in our focal populations emerge from the gravel, they spend a year in the stream (in the case of the Chinook salmon) or lake (sockeye) before migrating to the ocean. For sockeye salmon, growth in some streams is higher under warmer conditions (Schindler et al. 2005), although complex phenological changes in prey communities may not always benefit sockeye fry (Hampton et al. 2006). For Chinook salmon, survival during this period is lower under warmer- and lower-flow conditions (Crozier and Zabel 2006), which could increase the risk of extinction by 29–86% (Crozier et al. 2008). Potential evolutionary responses will depend on the mechanisms by which low fall flows and high summer temperatures reduce survival. Likely candidates include influences on growth rates and predation. Little is known about the evolutionary responses of juvenile salmon to changes in predation, so we here focus on growth.
Local adaptation of growth rate to water temperature does occur in at least some salmonines (Jensen et al. 2000; Finstad et al. 2004), notably after introduction to new environments (Haugen and Vollestad 2000; Quinn et al. 2001). Moreover, the contributions of body size and growth rate to survival in salmonids do appear to vary with environmental conditions (Zabel and Williams 2002; Zabel and Achord 2004). Although these patterns suggest growth rates can evolve in response to changing temperatures, there are several reasons for caution. First, the heritability of growth rate can be relatively low (0.04–0.3) in wild Chinook salmon (Hard 2004; de Leaniz et al. 2007; Carlson and Seamons 2008; Waples et al. 2008). Second, evolutionary responses are difficult to predict because growth rates are genetically correlated with many other traits under selection, such as egg size, agonistic behavior, age and size at smolting, and age and size at maturity (Hard 2004). Third, adaptation of growth rates to local temperatures appears strongest at low, rather than high, temperatures (Jensen et al. 2000). Finally, studies formally estimating natural selection in salmonid populations experiencing environmental change have not found strong selection on growth rate or body size (Hendry et al. 2003; Carlson et al. 2004). We tentatively conclude that climate-induced changes in growth rate are likely to be primarily plastic.
Downstream migration timing and early ocean stages
The periods of downstream migration and ocean entry are especially hazardous for salmon. Although many traits can influence survival during these periods, we focus on migration timing, which has been well studied and shows the potential for both plastic and genetic responses to climate change. The optimal timing of downstream migration, like that of upstream migration, reflects a trade-off between the time for growth before migration and the hazards of seasonally deteriorating river or ocean conditions. Smolt migration timing varies among populations (Peven 1987; Healey 1991; Orciari and Leonard 1996; Achord et al. 2001), but the relative contribution of genetic differences versus phenotypic plasticity to these patterns remains uncertain.
For our focal Chinook populations, survival during downstream migration is negatively correlated with temperatures over 13°C and positively correlated with flow (Achord et al. 2007). An earlier snowmelt and rising summer temperatures will cause unfavorable river conditions to occur earlier in summer, thus potentially favoring earlier migration. At present, salmon seem to be responding plastically by migrating earlier in years with warmer fall and spring temperatures (Achord et al. 2007), consistent with patterns seen in these species elsewhere (Quinn 2005). Phenotypic plasticity might thus accommodate climate change. However, with climate change, changes in conditions at the rearing location might not exactly parallel the changes in conditions in the lower river, estuary, and coastal environments. That is, earlier migration might well be adaptive with respect to survival in the upper river but not with respect to survival in the lower river or ocean. In such cases, the plastic response might not be adaptive and selection might favor a genetically based response. No studies have yet documented genetically based changes in smolt migration timing, but the trait appears to have a genetic basis (Stewart et al. 2006 and references therein).
Factors influencing the optimal timing of ocean entry are more difficult to predict but clearly important. Survival over the entire period of ocean residency is usually <4% for Snake River Chinook salmon (Williams et al. 2005), and most mortality is thought to occur within weeks to months of ocean entry (Pearcy 1992). Survival probabilities during this period are related to ocean conditions when the juveniles arrive (Logerwell et al. 2003; Scheuerell and Williams 2005; Zabel et al. 2006). Salmon grow quickly when upwelling winds bring cool, nutrient-rich water to the surface, stimulating the growth of plankton. Cooler water also reduces predation by displacing warm-water predators offshore. Some models predict that climate change will increase the intensity of upwelling but delay its onset (Snyder et al. 2003; Diffenbaugh et al. 2004). At present, naturally migrating smolts with earlier ocean entry usually have higher ocean survival, possibly reflecting maladaptation introduced by the effects of dams on migration speed (Zabel and Williams 2002; Waples et al. 2008). A delay in upwelling might improve the survival of late-entry smolts, ultimately selecting for later ocean entry. Later initiation of smolt migration or slower migration through the river would likely increase in-river mortality, thus setting the stage for climate change to impose contradictory selection on migration timing through in-river survival (favors earlier migration) and early-ocean survival (favors later migration). Other climate models predict that upwelling will instead shift earlier in the season (Hsieh and Boer 1992), in which case the two aspects of selection are instead complementary.
In this discussion, we have assumed that river conditions affect migration survival, and that arrival time in the estuary depends directly on migration date. At present, the vast majority of smolts (>80%) are, however, collected at upstream dams and taken downriver in barges. These fish can reach the estuary in 2 days instead of 2–6 weeks. Although earlier ocean entry in general appears advantageous for this population, barged fish typically have lower adult return rates than naturally migrating fish (Williams et al. 2005). The reasons for this difference are controversial (Budy et al. 2002; Muir et al. 2006). But regardless of the reasons, human actions greatly impact the selection pressures these fish experience, so it is misleading to consider potential evolutionary responses to climate change without considering our role (Waples et al. 2008).
Ocean residence
Most Columbia River salmon spend 1–4 years in the ocean, depending on environmental and genetic factors, so ocean conditions undoubtedly also impose selection. Ocean growth rates will likely respond to climate change through alterations in metabolic costs of foraging in a warmer ocean and shifts in prey abundance, composition, and distribution. We do not know enough either about how ocean food webs will respond to climate change, or how Chinook or sockeye salmon will respond to these changes to predict specific evolutionary consequences. Genetic variability in the migration patterns of salmon (Pascual and Quinn 1994; Kallio-Nyberg et al. 2000) represents the potential for adaptation of migration routes toward regions favorable for growth and survival. However, these processes are so poorly understood that it is difficult to speculate how rapidly adaptation might occur, and how it would interact with proximate responses to currents, temperature, food availability, and other stimuli.
Integrating across the complexity
In outlining the above suite of traits and life stages, we have attempted to assess how climate change might alter natural selection and drive evolutionary responses in a particular set of salmon populations (Table 1). We have highlighted interesting aspects of specific traits but have not considered interactions among them in detail. We now begin to address this complexity by proposing a conceptual model that integrates climate, plastic, and evolutionary effects across a particular life-history type, yearling juvenile and spring/summer adult migrating salmon. We focus on the timing of life-history events because phenology is likely to respond to climate change both evolutionarily and through plasticity (Bradshaw and Holzapfel 2008), and because changes in phenology at one life stage can directly affect phenology at other stages. We consider the timing of five major life-history events: upriver migration, spawning, emergence from the gravel, smolt migration and ocean entry. Note that we do not expect the following analysis to be correct in all respects, or to apply to all populations. Rather, we outline the possibilities and a framework as a basis for an integrated discussion.
Table 1.
Overview of some of the possible effects of climate change and potential plastic and evolutionary responses in Snake River spring/summer Chinook salmon.
| Climate change effect | Confidence in physical effect | Effect on fish | Plastic response | Potential evolutionary response | Reference |
|---|---|---|---|---|---|
| ↑ Peak summer and fall temperatures | High | ↓ Parr survival | Seek cooler refugia | Heat tolerance | Bisson and Davis (1976), McCullough (1999), Crozier and Zabel (2006)* |
| ↑ or ↓ growth, depending on food supply and fish density | ↑ Energetic efficiency at high temperatures | Brett et al. (1982), ISAB, (2007) | |||
| ↑ Predation on juveniles | Predator avoidance behavior, choosing suboptimal habitat | Petersen and Kitchell (2001)*, Marine and Cech (2004) | |||
| ↓ Adult survival due to stress from temperature or disease | Migration delays, higher stray rate | Earlier adult migration ↑ Disease resistance ↑ Energetic efficiency at high temperatures | McCullough (1999), Hyatt et al. (2003), Naughton et al. (2005), Goniea et al. (2006), Battin et al. (2007) | ||
| ↓ Reproductive success (↓ egg viability from thermal stress, or smaller eggs due to ↑ energetic cost) | Shift reproductive allocation | McCullough (1999), Kinnison et al. (2001), Rand et al. (2006) | |||
| ↓ Summer and fall flows | High | ↓ Parr survival | Change habitat | Crozier and Zabel (2006)* | |
| Shorter and milder winter | High | ↑ Development rates | Earlier emergence | ↑ Energetic efficiency at higher temperatures | Beacham and Murray (1989), Finstad et al. (2004) |
| ↑ Spring temperatures | High | ↑ Development rates | Earlier smolt migration | Beckman et al. (1998); Achord et al. (2007)* | |
| ↓ Smolt survival | ↑ Disease resistance earlier smolt migration ↑ Heat tolerance | Smith et al. (2003)* found no effect; Zabel et al. (in press)* negative effect | |||
| Earlier spring freshet | High | Earlier smolt migration | Achord et al. (2007)* | ||
| Weaker spring freshet | High | ↓ Smolt survival | Williams et al. (2005)*, Achord et al. (2007)* | ||
| ↓ Adult energetic cost (if not too hot) | Larger eggs | Kinnison et al. (2001), Rand et al. (2006) | |||
| Delay in upwelling | Low | ↑ Juvenile survival if arrival time does not change | Logerwell et al. (2003), Scheuerell and Williams (2005)*, Williams et al. (2005)*, Zabel et al. (2006)*, Waples et al. (2008)*) | ||
| ↑ Ocean stratification ↑ Surface temperature | High | ↑ Metabolic costs in surface water | ↑ Vertical migration Shift locations | ↓ Metabolic rates Change migration route | Welch et al. (1998), Walker et al. (2000), Portner and Knust (2007) |
| Acidification | High | ↓ Growth rate | Delayed maturation |
References with an * are specific to this population.
We start by assuming (Fig. 4, top panel) that, in a population, the peak of the phenotype distribution of timing (solid) for each life-history event coincides with the peak of the fitness function for that event (height of the dotted curve indicates the expected fitness of an individual with that timing phenotype). We therefore assume that salmon populations are locally adapted before climate change, such that the mean timing of each event approximates the optimal timing. It is certainly possible that the current populations are not adapted for the current conditions, given that the Columbia River has changed so dramatically and hatchery propagation and fisheries can exert countervailing selection. But attempting to integrate this possibility would mainly serve to complicate our illustration and is better left for a subsequent analysis.
Figure 4.
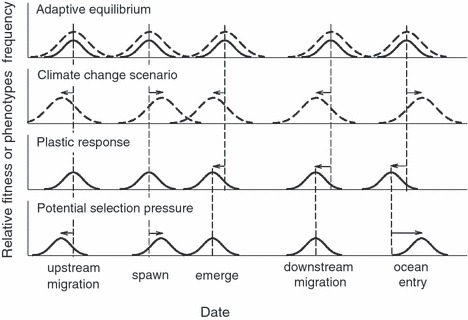
Hypothetical interaction between shifts in life-cycle timing and shifts in environmental optima. The x-axis (not to scale) represents the time from spawning through ocean entry. Solid bell curves represent the distribution of phenotypes and the dotted curves represent the relative fitness of these phenotypes in the current (top row) and climate-change (second row) conditions. Dashed vertical lines show the current phenotype. The line shifts represent plastic changes in phenology. The upper graph represents an equilibrium condition with the response of the fish adapted to environmental conditions. The second row shows how a hypothetical climate-change scenario might shift the optimal migration timing of each event. The third row shows the likely physiological (plastic) response to warmer temperatures phenology. The bottom row shows the potential evolutionary response to the mismatches depicted in the second and third rows. Note that earlier downstream migration but later ocean entry would seem to present contradictory pressures.
The second panel of Fig. 4 represents how fitness functions might shift in position under one potential climate-change scenario. First, an earlier onset of stressful temperatures shifts the optimum timing of upstream migration (note, however, that for populations tending to migrate after peak temperatures the optimum would shift later under warming conditions). Second, optimal spawning date will shift later in the year because warmer water will otherwise hasten egg development and cause the fry to emerge too early. Note that earlier adult migration but later spawning implies a longer stay in freshwater, which imposes energetic costs and higher risk of predation and thermal stress. For later spawning to be favored, the costs imposed on juvenile survival from early emergence must outweigh the costs imposed on adult survival and egg size. Furthermore, the shift in optimal spawning date will depend on the degree to which warmer water accelerates development more than it advances optimal emergence time, and the possibility of a plastic response in spawning date, which might be greater in this population than generally reported in the literature (Dan Isaac, pers. comm.). Third, optimal emergence timing should be earlier because warmer water should advance the date at which food becomes available. Fourth, excessively high river temperatures during the summer will advance the optimal timing of smolt migration, unless fifth, delayed upwelling along the coast delays optimal ocean entry. This combination illustrates a potential conflict between selections on life stages in different habitats: warm river temperatures will select for earlier migration, but ocean conditions might favor later migration.
The third panel shows the expected plastic response of each life-history event to climate change. We first expect migration and spawning date to remain largely unchanged owing to their low plasticity. We next expect earlier emergence timing because warmer incubation temperatures accelerate development (again note that the actual shift depends on any change in spawning date). Similarly, we expect earlier downstream migration because on an annual basis, smolting is advanced by earlier warming. Ocean entry is likely to advance because migration speeds typically accelerate in warmer water.
The fourth panel shows potential natural selection on the timing of each life-history event as a result of the mismatch between the new optimum and the phenotype distribution. First, we expect selection for earlier migration and later spawning because the optima shift with climate change, but the traits do not shift plastically. We next expect little selection on emergence or downstream migration timing because, although the optimum has advanced, the plastic response is in that direction. Finally, selection on ocean entry timing might be strong because the plastic shift in migration timing acted in the opposite direction from the new optimal.
This heuristic analysis illustrates the need for a closer examination of several key traits and stages. For example, selection on spawning date depends on at least three changes that are uncertain: (i) the advance in optimal emergence timing, (ii) a plastic change in spawning date owing to warmer waters, and (iii) potential costs of longer delays between migration and spawning. With regard to this last effect, advancing upstream migration dates and higher summer temperatures increase the length of time in freshwater during which energy stores are depleted, and cool-water refugia might contract, increasing prespawning mortality. This means that selection might not favor a delay in spawning date, but rather slower, embryo development rates. The lower heritabilities of embryo development rates would likely limit the selection response. As another example, consistent delays in the onset of upwelling would select very strongly to delay the time of ocean entry. Accordingly, selection might favor delayed onset of smolt migration or a slower migration, and yet both of these effects seem unlikely given that high summer temperatures during migration increase mortality rates. Under these conditions, selection might favor direct adaptations to resist the stresses associated with high temperatures or early ocean entry. Note that changes in upwelling timing are very uncertain, so this is not the only plausible scenario. Nonetheless, it does draw attention to a particular case where the plastic response in one stage might be unfavorable for the subsequent life stage.
Conclusions
Considerable uncertainty attends the prediction of evolutionary responses to climate warming (Holt 1990), even for a short-lived organism with a simple life cycle that is amenable to experiment (Etterson and Shaw 2001). The uncertainties are considerably greater for organisms such as salmon that have complex, migratory life cycles. Selection pressures might differ greatly in different life stages, and appropriate phenological cues are critical for successful transitions between habitats. For salmon, like most organisms, both plastic and evolutionary mechanisms will contribute to phenological changes. Moreover, the persistence of individual salmon populations through climate change will likely depend on the evolution of a variety of other, nonphenological traits as well.
We identified several traits with relatively high heritabilities, such as upstream migration date and spawning date, where we expect climate change to induce strong selection. Evolutionary responses in these traits are likely, as has been shown for other cases of environmental change influencing salmon (Hendry et al. 1998, 2000; Kinnison et al. 1998, 2008; Quinn et al. 2000, 2001) and for other organisms (reviews: Hendry and Kinnison 1999; Reznick and Ghalambor 2001). We identified other traits, such as emergence timing, smolt migration timing, and habitat choice, where phenotypic change might largely reflect plasticity. These plastic responses might often be adaptive and should greatly reduce mortality compared with selection acting on the same traits (Price et al. 2003; Ghalambor et al. 2007). However, more work is needed to assess how plasticity and evolutionary changes feed back to affect the productivity and persistence of populations (Kinnison and Hairston 2007; Kinnison et al. 2008).
Phenological changes are likely to be particularly important (see also Bradshaw and Holzapfel 2008). Indeed, some of the best evidence for phenotypic responses to environmental change are in the timing of migration or reproduction for salmon (Fig. 3, Quinn and Adams 1996) and for other organisms (Parmesan and Yohe 2003; Reale et al. 2003b; Parmesan 2006). Most of this evidence is currently observational, so it remains difficult to assess the relative contributions of genetic change versus plasticity (Gienapp et al. 2008). We argue that these contributions are likely to differ among various timing events, as has been observed for some birds (Both and Visser 2005). In salmon, changes in juvenile migration timing are likely to be mostly plastic, whereas changes in adult migration timing are likely to be mostly genetic. The norm of reaction that governs juvenile migration time might evolve over time, especially in response to changes in climate variability, but we do not yet have enough information to predict this process.
Although strong phenological responses to climate change are likely, they are not without constraint and might not obviate selection on other traits. For example, the life-history of salmon balances the timing of numerous events during transit from headwaters to the ocean and back again. Change in one aspect of timing might thus directly affect subsequent life-history stages, perhaps in maladaptive ways. If so, phenological changes might not sufficiently balance environmental changes, and selection might occur on other traits, such as disease resistance, metabolic responses to temperature, and the sensitivity of developmental processes to temperature. These traits often show less heritability, so evolutionary change will be slower. In general, changes in one trait, which might be plastic or genetic, will influence selection and evolutionary responses for other traits (Both and Visser 2001; Price et al. 2003; Ghalambor et al. 2007).
An important point to keep in mind is that in situ evolutionary change, while potentially saving distinctive populations from extirpation, might alter them so that they are no longer so distinctive. For example, Williams et al. (2008) argue that threatened Snake River fall Chinook salmon might be adapting to anthropogenic changes to their habitat by shifting from migration as subyearlings to migration as yearlings, thus gradually eliminating one of the dominant characteristics of the historical population. Ultimately, climate change might favor a change in the juvenile- and adult-migration phenology of Snake River spring/summer Chinook to the point that they no longer exhibit the northern ecotype of Chinook (Taylor 1990; Healey 1991; Brannon et al. 2004). Currently, fall Chinook salmon (with the typically southern ecotype) spawns in the lower Salmon River (StreamNet 2005). With climate change, some aspects of this phenotype might become more suitable at higher elevations, eventually encroaching on the habitat currently occupied by summer Chinook salmon. If genetic variation in the existing population is low, or immigration high, trait replacement might occur through gene flow rather than evolution in isolation, reducing the genetic distinctiveness of this population complex (Waples et al. 2004). Indeed, replacement by gene flow appears to have occurred in some populations of mice experiencing environmental change (Pergams and Lacy 2008). Such a scenario is complicated by the different spawning habitat preferences and ocean migration patterns of the two ecotypes, which might be tied to juvenile- or adult-migration timing. The linkages between and constraints on all these traits are not fully understood. Nonetheless, whether through in situ change or gene flow, evolutionary change induced by climate change might dramatically alter the structure and integrity of the evolutionarily significant units on which conservation designations are based.
How representative is our case study? The great diversity of salmon life histories precludes extending the details of our analysis too broadly. For example, some populations have a short freshwater residency but a long estuarine residency, which should shift the stage and environment where climate change is most likely to alter selection pressures. Furthermore, particular climate impacts not considered here will also have a profound impact on the evolution and long-term survival of Pacific salmon populations. For instance, winter flooding strongly influences egg survival (Schuett-Hames et al. 2000; Seiler et al. 2002, 2003) and is likely to increase extinction risk for some populations under climate change (Battin et al. 2007; ISAB 2007). Sea level rise, ocean acidification, changes in stream productivity, increased habitat availability at the northern end of the range, and myriad other anticipated and unanticipated effects of climate change will further complicate the evolutionary puzzle confronting salmon.
Regardless of the specific selective factors that will most affect a particular population, salmon in general will respond to climate change with a dynamic tension between phenological and nonphenological change, as well as interacting plastic and genetic shifts in phenotypes. These are the fundamental processes that require focused study in the near future. Integrated analyses have been useful in the study of squirrels (Reale et al. 2003a,b) and migratory birds (Both and Visser 2001, 2005; Nussey et al. 2005; Both and Marvelde 2007), and are likely to prove equally fruitful for salmon. Studies in wild salmon, in particular, are clearly needed, because most of the available genetic research has been conducted on hatchery fish (Carlson and Seamons 2008). Finally, because of the multiplicative impact of selection over the life cycle, it is crucial to consider the entire life cycle for species whose viability is at stake. A better understanding of the range of possible evolutionary responses to climate change is an essential component of effective, flexible strategies for the conservation of organisms with complex life histories, such as salmon.
Acknowledgments
This paper resulted from interactions at an Evolutionary Workshop held in Seattle, December 2006. We deeply thank Robin Waples, who organized the workshop, and Bill Bradshaw who also helped develop these ideas. We also thank Jeff Hard, Kym Jacobson, Rich Zabel, and John Williams for their insightful suggestions that improved the manuscript.
Literature cited
- Achord S, Axel G, Hockersmith E, Sandford B, Eppard EB, Mathews GM. Monitoring the migration of wild Snake River spring/summer Chinook salmon smolts, 2000. Report to the U.S. Department of Energy, Bonneville Power Administration. 2001. Portland, Oregon. http://www.efw.bpa.gov/Publications/
- Achord S, Zabel RW, Sandford BP. Migration timing, growth, and estimated parr-to-smolt survival rates of wild Snake River spring-summer Chinook salmon from the Salmon River basin, Idaho, to the Lower Snake River. Transactions of the American Fisheries Society. 2007;136:142–154. [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection – theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Augerot X. Atlas of Pacific Salmon: the First Map-Based Status Assessment of Salmon in the North Pacific. Berkeley, CA: University of California Press; 2005. [Google Scholar]
- Balanya J, Oller JM, Huey RB, Gilchrist GW, Serra L. Global genetic change tracks global climate warming in Drosophila subobscura. Science. 2006;313:1773–1775. doi: 10.1126/science.1131002. [DOI] [PubMed] [Google Scholar]
- Bartholomew JL. Host resistance to infection by the myxosporean parasite Ceratomyxa shasta: a review. Journal of Aquatic Animal Health. 1998;10:112–120. [Google Scholar]
- Battin J, Wiley MW, Ruckelshaus MH, Palmer RN, Korb E, Bartz KK, Imaki H. Projected impacts of climate change on salmon habitat restoration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6720–6725. doi: 10.1073/pnas.0701685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham TD, Evelyn TPT. Population and genetic variation in resistance of Chinook salmon to vibriosis, furunculosis, and bacterial kidney disease. Journal of Aquatic Animal Health. 1992;4:153–167. [Google Scholar]
- Beacham TD, Murray CB. Variation in developmental biology of sockeye salmon (Oncorhynchus nerka) and Chinook salmon (O. tshawytscha) in British Columbia. Canadian Journal of Zoology. 1989;67:2081–2089. [Google Scholar]
- Beacham TD, Murray CB. Temperature, egg size, and development of embryos and alevins of five species of Pacific salmon: a comparative analysis. Transactions of the American Fisheries Society. 1990;119:927–945. [Google Scholar]
- Beacham TD, Withler RE. Genetic variation in mortality of Chinook salmon, Oncorhynchus tshawytscha (Walbaum), challenged with high water temperatures. Aquaculture and Fisheries Management. 1991;22:125–133. [Google Scholar]
- Beckman BR, Larsen DA, Lee-Pawlak B, Dickhoff WW. Relation of fish size and growth rate to migration of spring Chinook salmon smolts. North American Journal of Fisheries Management. 1998;18:537–546. [Google Scholar]
- Beer WN, Anderson JJ. Effect of spawning day and temperature on salmon emergence: interpretations of a growth model for Methow River Chinook. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:943–949. [Google Scholar]
- Berman CH, Quinn TP. Behavioural thermoregulation and homing by spring Chinook salmon, Oncorhynchus tshawytscha (Walbaum), in the Yakima River. Journal of Fish Biology. 1991;39:301–312. [Google Scholar]
- Berteaux D, Reale D, McAdam A, Boutin S. Keeping pace with fast climate change: can Arctic life count on evolution? Integrative and Comparative Biology. 2004;44:140–151. doi: 10.1093/icb/44.2.140. [DOI] [PubMed] [Google Scholar]
- Bisson PA, Davis GE. Production of juvenile Chinook salmon, Oncorhynchus tshawytscha, in a heated model stream. Fishery Bulletin. 1976;74:763–774. [Google Scholar]
- Both C, Te Marvelde L. Climate change and timing of avian breeding and migration throughout Europe. Climate Research. 2007;35:93–105. [Google Scholar]
- Both C, Visser ME. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. [DOI] [PubMed] [Google Scholar]
- Both C, Visser ME. The effect of climate change on the correlation between avian life-history traits. Global Change Biology. 2005;11:1606–1613. [Google Scholar]
- Bower SM, Withler RE, Riddell BE. Genetic variation in resistance to the hemoflagellate Cryptobia salmositica in coho and sockeye salmon. Journal of Aquatic Animal Health. 1995;7:185–194. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Genetic response to rapid climate change: it’s seasonal timing that matters. Molecular Ecology. 2008;17:157–166. doi: 10.1111/j.1365-294X.2007.03509.x. [DOI] [PubMed] [Google Scholar]
- Brannon EL. Mechanisms stabilizing salmonid fry emergence timing. Can. Spec. Pub. Fish. Aquat. Sci. 1987;96:120–124. [Google Scholar]
- Brannon EL, Powell MS, Quinn TP, Talbot A. Population structure of Columbia River Basin Chinook salmon and steelhead trout. Reviews in Fisheries Science. 2004;12:99–232. [Google Scholar]
- Brett JR. Some principles of the thermal requirements of fishes. Quarterly Review of Biology. 1956;31:75–87. [Google Scholar]
- Brett JR, Charke WC, Shelbourn JE. Experiments on thermal requirements for growth and food conversion efficiency of juvenile Chinook salmon, Oncorhynchus tshawytscha. Canadian Technical Report of Fisheries Aquatic Sciences. 1982;1127:1–33. [Google Scholar]
- Budy P, Thiede GP, Bouwes N, Petrosky CE, Schaller H. Evidence linking delayed mortality of Snake River salmon to their earlier hydrosystem experience. North American Journal of Fisheries Management. 2002;22:35–51. [Google Scholar]
- Bürger R, Lynch M. Evolution and extinction in a changing environment: a quantitative-genetic analysis. Evolution. 1995;49:151–163. doi: 10.1111/j.1558-5646.1995.tb05967.x. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Seamons TR. A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evolutionary Applications. 2008;1:222–238. doi: 10.1111/j.1752-4571.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Hendry AP, Letcher BH. Natural selection acting on body size, growth rate and compensatory growth: an empirical test in a wild trout population. Evolutionary Ecology Research. 2004;6:955–973. [Google Scholar]
- Chapman PF. Occurrence of the noninfective stage of Ceratomyxa shasta in mature summer Chinook salmon in the South Forth Salmon River, Idaho. Progressive Fish-Culturist. 1986;48:304–306. [Google Scholar]
- Crozier LG, Zabel RW. Climate impacts at multiple scales: evidence for differential population responses in juvenile Chinook salmon. Journal of Animal Ecology. 2006;75:1100–1109. doi: 10.1111/j.1365-2656.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- Crozier LG, Zabel RW, Hamlet AF. Predicting differential effects of climate change at the population level with life-cycle models of spring Chinook salmon. Global Change Biology. 2008;14:236–249. [Google Scholar]
- DART (Data Access in Real Time) 2007. Columbia River data access in real time http://www.cbr.washington.edu/dart/dart.html (accessed June 2007)
- Davis MB, Shaw RG, Etterson JR. Evolutionary responses to changing climate. Ecology. 2005;86:1704–1714. [Google Scholar]
- Diffenbaugh NS, Snyder MA, Sloan LC. Could CO2-induced land-cover feedbacks alter near-shore upwelling regimes? Proceedings of the National Academy of Sciences of the United States of America. 2004;101:27–32. doi: 10.1073/pnas.0305746101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato M. A statistical model for estimating stream temperatures in the Salmon and Clearwater River Basins, central Idaho, Water-Resources Investigations Report 02-4195. Boise, Idaho: U.S. Department of the Interior, U.S. Geological Survey, Idaho Department of Environmental Quality; 2002. http://id.water.usgs.gov/PDF/wri024195/index.html. [Google Scholar]
- Einum S, Fleming IA. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar. Evolution. 2000;54:628–639. doi: 10.1111/j.0014-3820.2000.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Etterson JR, Shaw RG. Constraint to adaptive evolution in response to global warming. Science. 2001;294:151–154. doi: 10.1126/science.1063656. [DOI] [PubMed] [Google Scholar]
- Finstad AG, Forseth T, Faenstad TF, Ugedal O. The importance of ice cover for energy turnover in juvenile Atlantic salmon. Journal of Animal Ecology. 2004;73:959–966. [Google Scholar]
- Garland T, Adolph SC. Physiological differentiation of vertebrate populations. Annual Review of Ecology and Systematics. 1991;22:193–228. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. [Google Scholar]
- Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. Climate change and evolution: disentangling environmental and genetic responses. Molecular Ecology. 2008;17:167–178. doi: 10.1111/j.1365-294X.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- Goniea TM, Keefer ML, Bjornn TC, Peery CA, Bennett DH, Stuehrenberg LC. Behavioral thermoregulation and slowed migration by adult fall Chinook salmon in response to high Columbia River water temperatures. Transactions of the American Fisheries Society. 2006;135:408–419. [Google Scholar]
- Good TP, Waples RS, Adams P. Updated status of federally listed ESUs of West Coast salmon and steelhead. Seattle, WA: U.S. Department of Commerce; 2005. NOAA Tech. Memo. NMFS-NWFSC-66. http://www.nwfsc.noaa.gov/publications/ [Google Scholar]
- Groot C, Margolis L. Pacific Salmon Life Histories. Vancouver: UBC Press; 1991. [Google Scholar]
- Hampton SE, Romare P, Seiler DE. Environmentally controlled Daphnia spring increase with implications for sockeye salmon fry in Lake Washington, USA. Journal of Plankton Research. 2006;28:399–406. [Google Scholar]
- Hard JJ. Evolution of Chinook salmon life history under size-selective harvest. In: Hendry AP, Stearns S, editors. Evolution Illuminated: Salmon and Their Relatives. Oxford, UK: Oxford University Press; 2004. pp. 315–337. [Google Scholar]
- Hard JJ, Elliott DG, Pascho RJ, Chase DM, Park LK, Winton JR, Campton DE. Genetic effects of ELISA-based segregation for control of bacterial kidney disease in Chinook salmon (Oncorhynchus tshawytscha. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:2793–2808. [Google Scholar]
- Harrington R, Woiwod I, Sparks T. Climate change and trophic interactions. Trends in Ecology and Evolution. 1999;14:146–150. doi: 10.1016/s0169-5347(99)01604-3. [DOI] [PubMed] [Google Scholar]
- Haugen TO, Vollestad LA. Population differences in early life-history traits in grayling. Journal of Evolutionary Biology. 2000;13:897–905. [Google Scholar]
- Healey MC. Life history of Chinook salmon (Oncorhynchus tshawytscha. In: Groot C, Margolis L, editors. Pacific Salmon Life Histories. Vancouver: UBC Press; 1991. pp. 313–393. [Google Scholar]
- Hebert KP, Goddard PL, Smoker WW, Gharrett AJ. Quantitative genetic variation and genotype by environment interaction of embryo development rate in pink salmon (Oncorhynchus gorbuscha. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:2048–2057. [Google Scholar]
- Hendry AP, Day T. Population structure attributable to reproductive time: isolation by time and adaptation by time. Molecular Ecology. 2005;14:901–916. doi: 10.1111/j.1365-294X.2005.02480.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Kinnison MT. Perspective: the pace of modern life: measuring rates of contemporary microevolution. Evolution. 1999;53:1637–1653. doi: 10.1111/j.1558-5646.1999.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Hensleigh JE, Reisenbichler RR. Incubation temperature, developmental biology, and the divergence of sockeye salmon (Oncorhynchus nerka) within Lake Washington. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1387–1394. [Google Scholar]
- Hendry AP, Wenburg JK, Bentzen P, Volk EC, Quinn TP. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Hendry AP, Letcher BH, Gries G. Estimating natural selection acting on stream-dwelling Atlantic salmon: implications for the restoration of extirpated populations. Conservation Biology. 2003;17:795–805. [Google Scholar]
- High B, Peery CA, Bennett DH. Temporary staging of Columbia River summer steelhead in coolwater areas and its effect on migration rates. Transactions of the American Fisheries Society. 2006;135:519–528. [Google Scholar]
- Hill JK, Thomas CD, Blakeley DS. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia. 1999;121:165–170. doi: 10.1007/s004420050918. [DOI] [PubMed] [Google Scholar]
- Hinch SG, Rand PS. Swim speeds and energy use of upriver-migrating sockeye salmon (Oncorhynchus nerka): role of local environment and fish characteristics. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1821–1831. [Google Scholar]
- Hodgson S, Quinn TP. The timing of adult sockeye salmon migration into fresh water: adaptations by populations to prevailing thermal regimes. Canadian Journal of Zoology. 2002;80:542–555. [Google Scholar]
- Hodgson S, Quinn TP, Hilborn R, Francis RC, Rogers DE. Marine and freshwater climatic factors affecting interannual variation in the timing of return migration to freshwater of sockeye salmon (Oncorhynchus nerka. Fisheries Oceanography. 2006;15:1–24. [Google Scholar]
- Holt RD. The microevolution consequences of climate change. TREE. 1990;5:311–315. doi: 10.1016/0169-5347(90)90088-U. [DOI] [PubMed] [Google Scholar]
- Hsieh WW, Boer GJ. Global climate change and ocean upwelling. Fisheries Oceanography. 1992;1:333–338. [Google Scholar]
- Hutchings JA. Norms of reaction and phenotypic plasticity in salmonid life histories. In: Hendry AP, Stearns S, editors. Evolution Illuminated: Salmon and Their Relatives. New York: Oxford University Press; 2004. pp. 154–176. [Google Scholar]
- Hyatt KD, Stockwell MM, Rankin DP. Impact and adaptation responses of Okanagan River sockeye salmon (Oncorhynchus nerka) to climate variation and change effects during freshwater migration: stock restoration and fisheries management implications. Canadian Water Resources Journal. 2003;28:689–713. [Google Scholar]
- ISAB (Independent Scientific Advisory Board) Climate change impacts on Columbia River Basin fish and wildlife. Portland, Oregon: Northwest Power and Conservation Council, Columbia River Basin Indian Tribes, National Marine Fisheries Service; 2007. p. 136. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Climate Change 2007: The Physical Science Basis: Intergovernmental Panel on Climate Change Fourth Assessment Report. 2007. http://www.ipcc.ch/ (accessed March 2007)
- Jensen AJ, Forseth T, Johnsen BO. Latitudinal variation in growth of young brown trout Salmo trutta. Journal of Animal Ecology. 2000;69:1010–1020. [Google Scholar]
- Kallio-Nyberg I, Koljonen M-L, Saloniemi I. Effect of maternal and paternal line on spatial and temporal marine distribution in Atlantic salmon. Animal Behaviour. 2000;60:377. doi: 10.1006/anbe.2000.1465. [DOI] [PubMed] [Google Scholar]
- Keefer ML, Peery CA, Jepson MA, Tolotti KR, Bjornn TC. Stock-specific migration timing of adult spring–summer Chinook salmon in the Columbia River Basin. North American Journal of Fisheries Management. 2004;24:1145–1162. [Google Scholar]
- Kinnison M, Hairston N., Jr Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Functional Ecology. 2007;21:441–454. [Google Scholar]
- Kinnison MT, Unwin MJ, Hershberger WK, Quinn TP. Egg size, fecundity, and development rate of two introduced New Zealand Chinook salmon (Oncorhynchus tshawytscha) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:1946–1953. [Google Scholar]
- Kinnison MT, Unwin MJ, Hendry AP, Quinn TP. Migratory costs and the evolution of egg size and number in introduced and indigenous salmon populations. Evolution. 2001;55:1656–1667. doi: 10.1111/j.0014-3820.2001.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Kinnison MT, Unwin MJ, Quinn TP. Eco-evolutionary vs. habitat contributions to invasion in salmon: experimental evaluation in the wild. Molecular Ecology. 2008;17:405–414. doi: 10.1111/j.1365-294X.2007.03495.x. [DOI] [PubMed] [Google Scholar]
- Konecki JT, Woody CA, Quinn TP. Critical thermal maxima of coho salmon (Oncorhynchus kisutch) fry under field and laboratory acclimation regimes. Canadian Journal of Zoology. 1995a;73:993–996. [Google Scholar]
- Konecki JT, Woody CA, Quinn TP. Temperature preference in two populations of juvenile coho salmon (Oncorhynchus kisutch) Env. Journal of Fish Biology. 1995b;44:417–421. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- De Leaniz CG, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, Aubin-Horth N, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biological Reviews. 2007;82:173–211. doi: 10.1111/j.1469-185X.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Letcher BH, Dubreuil T, O’Donnell MJ, Obedzinski M, Griswold K, Nislow KH. Long-term consequences of variation in timing and manner of fry introduction on juvenile Atlantic salmon (Salmo salar) growth, survival, and life-history expression. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:2288–2301. [Google Scholar]
- Logerwell EA, Mantua NJ, Lawson PW, Francis RC, Agostini VN. Tracking environmental processes in the coastal zone for understanding and predicting Oregon coho (Oncorhynchus kisutch) marine survival. Fisheries Oceanography. 2003;16:554–568. [Google Scholar]
- Lynch M. Estimating genetic correlations in natural populations. Genetical Research. 1999;74:255–264. doi: 10.1017/s0016672399004243. [DOI] [PubMed] [Google Scholar]
- Lynch M, Lande R. Evolution and extinction in response to environmental change. In: Kareiva P, Kingsolver J, Huey R, editors. Biotic Interactions and Global Change. Sunderland, MA: Sinauer Ass. Inc; 1993. pp. 234–251. [Google Scholar]
- Marcogliese DJ. Implications of climate change for parasitism of animals in the aquatic environment. Canadian Journal of Zoology. 2001;79:1331–1352. [Google Scholar]
- Marine KR, Cech JJ. Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook salmon. North American Journal of Fisheries Management. 2004;24:198–210. [Google Scholar]
- Materna E. Seattle, WA: U.S. Fish and Wildlife Service; 2001. Temperature interaction, Issue Paper 4. Temperature Water Quality Criteria Guidance Development Project, EPA-910-D-01-004, Environmental Protection Agency Region 10. [Google Scholar]
- McClure M, Holmes EE, Sanderson BL, Jordan CE. A large-scale multispecies status assessment: anadromous salmonids in the Columbia River basin. Ecological Applications. 2003;13:964–989. [Google Scholar]
- McCullough DA. A Review and Synthesis of Effects of Alterations to the Water Temperature Regime on Freshwater Life Stages of Salmonids, with Special Reference to Chinook Salmon. Seattle, Washington: U.S. Environmental Protection Agency, Region 10; 1999. [Google Scholar]
- Miller MP, Vincent RE. Rapid natural selection for resistance to an introduced parasite of rainbow trout. Evolutionary Applications. 2008;1:336–341. doi: 10.1111/j.1752-4571.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WD, Marsh DM, Sandford B, Smith SG, Williams JG. Post-hydropower system delayed mortality of transported Snake River stream-type Chinook salmon: unraveling the mystery. Transactions of the American Fisheries Society. 2006;135:1523–1534. [Google Scholar]
- Naughton GP, Caudill CC, Keefer ML, Bjornn TC, Stuehrenberg LC, Peery CA. Late-season mortality during migration of radio-tagged adult sockeye salmon (Oncorhynchus nerka) in the Columbia River. Canadian Journal of Fisheries and Aquatic Sciences. 2005;62:30–47. [Google Scholar]
- NMFS. Endandered and threatened species: threatened status for Snake River spring/summer Chinook salmon. Federal Register. 1992;57:14653–14662. [Google Scholar]
- Nussey DH, Postma E, Gienapp P, Visser M. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–307. doi: 10.1126/science.1117004. [DOI] [PubMed] [Google Scholar]
- Orciari RD, Leonard GH. Length characteristics of smolts and timing of downstream migration among three strains of Atlantic salmon in a southern New England stream. North American Journal of Fisheries Management. 1996;16:851–860. [Google Scholar]
- Ordal EJ, Pacha RE. Proceedings of the Twelfth Pacific Department of Environmental Quality, Standard and Assessment Section. Final issues papers. 1963. The effects of temperature on disease in fish; pp. 39–56. Portland, Oregon. [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics. 2006;37:637–669. [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pascual MA, Quinn TP. Geographical patterns of straying of fall Chinook salmon (Oncorhynchus tshawytscha) from Columbia River (U.S.A.) hatcheries. Aquaculture and Fisheries Management. 1994;25:17–30. [Google Scholar]
- Pearcy WG. Ocean Ecology of North Pacific Salmonids. Seattle, Washington: University of Washington Press; 1992. [Google Scholar]
- Pergams ORW, Lacy RC. Rapid morphological and genetic change in Chicago-area Peromyscus. Molecular Ecology. 2008;17:450–463. doi: 10.1111/j.1365-294X.2007.03517.x. [DOI] [PubMed] [Google Scholar]
- Petersen JH, Kitchell JF. Climate regimes and water temperature changes in the Columbia River: bioenergetic implications for predators of juvenile salmon. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:1831–1841. [Google Scholar]
- Peven CM. Downstream migration timing of two stocks of sockeye salmon on the Mid-Columbia River. Northwest Science. 1987;61:186–190. [Google Scholar]
- Portner HO, Knust R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science. 2007;315:95–97. doi: 10.1126/science.1135471. [DOI] [PubMed] [Google Scholar]
- Price TD, Qvarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London Series B – Biological Sciences. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout T. The relation between fitness components and population prediction in Drosophila. I: the estimation of fitness components. Genetics. 1971;68:127–149. doi: 10.1093/genetics/68.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TP. The Behavior and Ecology of Pacific Salmon and Trout. Seattle, Washington: University of Washington Press; 2005. [Google Scholar]
- Quinn TP, Adams DJ. Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology. 1996;77:1151–1162. [Google Scholar]
- Quinn TP, Unwin MJ, Kinnisona MT. Evolution of temporal isolation in the wild: genetic divergence in timing of migration and breeding by introduced Chinook salmon populations. Evolution. 2000;54:1372–1385. doi: 10.1111/j.0014-3820.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Quinn TP, Kinnison MT, Unwin MJ. Evolution of Chinook salmon (Oncorhynchus tshawytscha) populations in New Zealand: pattern, rate, and process. Genetica. 2001;112-113:493–513. [PubMed] [Google Scholar]
- Quinn TP, Peterson JA, Gallucci VF, Hershberger WK, Brannon EL. Artificial selection and environmental change: countervailing factors affecting the timing of spawning by coho and Chinook salmon. Transactions of the American Fisheries Society. 2002;131:591–598. [Google Scholar]
- Rand PS, Hinch SG, Morrison J, Foreman MGG, MacNutt MJ, Macdonald JS, Healey MC, et al. Effects of river discharge, temperature, and future climates on energetics and mortality of adult migrating Fraser River sockeye salmon. Transactions of the American Fisheries Society. 2006;135:655–667. [Google Scholar]
- Reale D, Berteaux D, McAdam A, Boutin S. Life-time selection on heritable life-history traits in a natural population of red squirrels. Evolution. 2003a;57:2416–2423. doi: 10.1111/j.0014-3820.2003.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Reale D, McAdam A, Boutin S, Berteaux D. Genetic and plastic responses of a northern mammal to climate change. Proceedings of the Royal Society of London B – Biological Sciences. 2003b;270:591–596. doi: 10.1098/rspb.2002.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch TB, Wood TE. Molecular ecology of global change. Molecular Ecology. 2007;16:3973–3992. doi: 10.1111/j.1365-294X.2007.03454.x. [DOI] [PubMed] [Google Scholar]
- Reznick DN, Ghalambor CK. The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica. 2001;112:183–198. [PubMed] [Google Scholar]
- Ricker WE. Hereditary and environmental factors affecting certain salmonid populations. In: Simon RC, Larkin PA, editors. The Stock Concept in Pacific Salmon. H.R. MacMillan Lectures in Fisheries. Vancouver: University of British Columbia; 1972. pp. 19–160. [Google Scholar]
- Sala OE, Chapin FS, III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Salathé EP. Influences of a shift in North Pacific storm tracks on western North American precipitation under global warming. Geophysical Research Letters. 2006;33:L19820. [Google Scholar]
- Salinger DH, Anderson JJ. Effects of water temperature and flow on adult salmon migration swim speed and delay. Transactions of the American Fisheries Society. 2006;135:188–199. [Google Scholar]
- Scheuerell MD, Williams JG. Forecasting climate-induced changes in the survival of Snake River spring/summer Chinook salmon. Fisheries Oceanography. 2005;14:448–457. [Google Scholar]
- Schindler DE, Rogers DE, Scheuerell MD, Abrey CA. Effects of changing climate on zooplankton and juvenile sockeye salmon growth in southwestern Alaska. Ecology. 2005;86:198–209. [Google Scholar]
- Schuett-Hames DE, Peterson NP, Conrad R, Quinn TP. Patterns of gravel scour and fill after spawning by chum salmon in a Western Washington stream. North American Journal of Fisheries Management. 2000;20:610–617. [Google Scholar]
- Seiler DE, Volkhardt G, Fleischer L, Kishimoto L. 2000 Cedar River Sockeye Salmon Fry Production Evaluation. Olympia, WA: Washington Department of Fish and Wildlife; 2002. [Google Scholar]
- Seiler DE, Neuhauser S, Kishimoto L. 2002 Skagit River 0+ Chinook Production Evaluation. Olympia, WA: Washington Department of Fish and Wildlife; 2003. [Google Scholar]
- Smith SG, Muir WD, Hockersmith EE, Zabel RW, Graves RJ, Ross CV, Connor WP, et al. Influence of river conditions on survival and travel time of Snake River subyearling fall Chinook salmon. North American Journal of Fisheries Management. 2003;23:939–961. [Google Scholar]
- Snyder MA, Sloan LC, Diffenbaugh NS, Bell JL. Future climate change and upwelling in the California Current. Geophysical Research Letters. 2003;30 ARTN 1823. [Google Scholar]
- Stewart IT, Cayan DR, Dettinger MD. Changes toward earlier streamflow timing across western North America. Journal of Climate. 2005;18:1136–1155. [Google Scholar]
- Stewart DC, Middlemas SJ, Youngson AF. Population structuring in Atlantic salmon (Salmo salar): evidence of genetic influence on the timing of smolt migration in sub-catchment stocks. Ecology of Freshwater Fish. 2006;15:552–558. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution. 2003;18:94–101. [Google Scholar]
- StreamNet. 2005. Fish data for the Northwest. http://www.streamnet.org/ (accessed January 2005)
- Tallman RF. Genetic differentiation among seasonally distinct spawning populations of chum salmon, Oncorhynchus keta. Aquaculture. 1986;57:211–217. [Google Scholar]
- Tallman RF, Healey MC. Phenotypic differentiation in seasonal ecotypes of chum salmon, Oncorhynchus keta. Canadian Journal of Fisheries and Aquatic Sciences. 1991;48:661–671. [Google Scholar]
- Taylor EB. Environmental correlates of life-history variation in juvenile Chinook salmon, Oncorhynchus tshawytscha (Walbaum) Journal of Fish Biology. 1990;37:1–17. [Google Scholar]
- Taylor EB. A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture. 1991;98:185–207. [Google Scholar]
- Thomas C, Bodsworth E, Wilson R, Simmons A, Davies Z, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thomas C, Cameron A, Green R, Bakkenes M, Beaumont L, Collingham Y, Erasmus B, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tiffan KD, Garland RD, Wagner PG. Osmoregulatory performance, migration behavior, and marking of subyearling Chinook salmon at McNary Dam to estimate adult contribution. In: Rondorf DW, Tiffan KG, editors. Identification of the spawning, rearing, and migratory requirement of fall Chinook salmon in the Columbia River basin. 1994 Report to Bonneville Power Administration. Portland, OR: U. S. Dept. of Energy; 1996. [Google Scholar]
- Torgersen CE, Price DM, Li HW, McIntosh BA. Multi-scale thermal refugia and stream habitat associations of Chinook salmon in northeastern Oregon. Ecological Applications. 1999;9:301–319. [Google Scholar]
- Unwin MJ, Quinn TP, Kinnison MT, Boustead NC. Divergence in juvenile growth and life history in two recently colonized and partially isolated Chinook salmon populations. Journal of Fish Biology. 2000;57:943–960. [Google Scholar]
- Visser M, Both C. Review. Shifts in phenology due to global climate change: the need for a yardstick. Proceedings of the Royal Society of London B – Biological Sciences. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RV, Myers KW, Davis ND, Aydin KY, Friedland KD, Carlson HR, Boehlert GW, et al. Diurnal variation in thermal environment experienced by salmonids in the North Pacific as indicated by data storage tags. Fisheries Oceanography. 2000;9:171–186. [Google Scholar]
- Waples RS, Teel DJ, Myers JM, Marshall AR. Life-history divergence in Chinook salmon: historic contingency and parallel evolution. Evolution. 2004;58:386–403. [PubMed] [Google Scholar]
- Waples RS, Zabel RW, Scheuerell MD, Sanderson BL. Evolutionary responses by native species to major anthropogenic changes to their ecosystems: Pacific salmon in the Columbia River hydropower system. Molecular Ecology. 2008;17:84–96. doi: 10.1111/j.1365-294x.2007.03510.x. [DOI] [PubMed] [Google Scholar]
- Welch DW, Ishida Y, Nagasawa K. Thermal limits and ocean migrations of sockeye salmon (Oncorhynchus nerka): long-term consequences of global warming. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:937–948. [Google Scholar]
- Williams JG, Smith SG, Zabel RW, Muir WD, Scheuerell MD, Sandford BP, Marsh DM, et al. Effects of the Federal Columbia River Power System on salmonid populations. Seattle, WA: U.S. Dept of Commerce; 2005. Tech Memo Number: NMFS-NWFSC-63 Document ID: 6061. http://www.nwfsc.noaa.gov/ [Google Scholar]
- Williams JG, Zabel RW, Waples RS, Hutchings JA, Connor WP. Potential for anthropogenic disturbances to influence evolutionary change in the life history of a threatened salmonid. Evolutionary Applications. 2008;1:271–285. doi: 10.1111/j.1752-4571.2008.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Hinch SG, Cooke SJ, Crossin GT, Patterson DA, Farrell AP, Van der Kraak G, et al. Physiological and energetic correlates of en route mortality for abnormally early migrating adult sockeye salmon (Oncorhynchus nerka) in the Thompson River, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:1067–1077. [Google Scholar]
- Zabel RW, Achord S. Relating size of juveniles to survival within and among populations of Chinook salmon. Ecology. 2004;85:795–806. [Google Scholar]
- Zabel RW, Williams JG. Selective mortality in Chinook salmon: what is the role of human disturbance? Ecological Applications. 2002;12:173–183. [Google Scholar]
- Zabel RW, Scheuerell MD, McClure MM, Williams JG. The interplay between climate variability and density dependence in the population viability of Chinook salmon. Conservation Biology. 2006;20:190–200. doi: 10.1111/j.1523-1739.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- Zabel RW, Faulkner J, Smith SG. Comprehensive Passage (COMPASS) Model: a model of downstream migration and survival of juvenile salmonids through a hydropower system. Hydrobiologia. in press. [Google Scholar]
- Zinn JL, Johnson KA, Sanders JE, Fryer JL. Susceptibility of salmonid species and hatchery strains of Chinook salmon (Oncorhynchus tshawytscha) to infections by Ceratomyxa shasta. Journal of the Fisheries Research Board of Canada. 1977;34:933–936. [Google Scholar]


