Abstract
Despite the optimism of some molecular biologists, natural selection among the wild ancestors of crops is unlikely to have missed simple genetic improvements that would consistently have enhanced individual fitness. Tradeoff-free opportunities for further improvement of crop traits like photosynthetic efficiency or drought tolerance may therefore be elusive. Opportunities linked to acceptable tradeoffs may be abundant, however. Tradeoffs between individual competitiveness and the collective productivity of plant communities (e.g. those linked to height) have been key to past increases in yield potential. Solar tracking by leaves could involve such tradeoffs, if photosynthetic benefits to tracking leaves are outweighed by increased shading of leaves lower in the canopy. This hypothesis was tested using rotation in the horizontal plane to disrupt solar tracking in alfalfa. In sparse canopies, solar tracking increased net canopy photosynthesis, but rarely by more than 3%. As leaf area increased, solar tracking tended to decrease net canopy photosynthesis, despite edge effects in our 1-m2 artificial communities, which probably exaggerated net photosynthetic benefits of tracking. Computer modeling suggested that the season-long effects of solar tracking on community productivity can be negative. Solar tracking may have persisted, nonetheless, because individuals whose leaves track the sun increase shading of competitors.
Keywords: competition, heliotropism, photosynthesis, solar tracking, tradeoff
Introduction
Molecular biologists often identify specific genes important in crop growth or stress tolerance. Increasing the expression of such genes is now a popular approach for genetic improvement of crops. But natural selection among the wild ancestors of crops is unlikely to have missed simple genetic improvements that would consistently have enhanced individual fitness under past conditions (Denison et al. 2003). Any constitutive increase in expression of a gene for drought tolerance, for example, would almost certainly regenerate a trait repeatedly rejected by past natural selection, presumably due to tradeoffs. Increased expression of a gene that is beneficial under drought might, for example, decrease growth under well-watered conditions. This could be the case, for example, for a highly publicized ‘drought-tolerant’ maize cultivar, for which yield data were reported only under a particular drought treatment (Nelson et al. 2007). Natural selection could have rejected this option for other reasons, of course, such as tradeoffs with competitiveness.
A genotype that is radically different from anything existing today may never have been tested by natural selection, so we can not assume it was rejected due to tradeoffs. But producing such genotypes is likely to require more-complex genetic changes than the evolution of C4 photosynthesis, which natural selection has achieved repeatedly (Kellogg 1999). Unfortunately, our present scientific ability to predict all of the field-level consequences of such complex genetic changes is even more limited than our technical ability to implement them.
Genetic improvement of crop yield potential by humans has usually involved tradeoffs rejected by past natural selection but acceptable to us (Denison 2009), although these tradeoffs have not always been obvious. Sometimes, the tradeoff is between adaptation to past and present conditions. A crop grown at a new latitude may need different photoperiod responses to complete seed development before winter. Irrigation or fertilizer use can create new opportunities for genetic improvements linked to tradeoffs between root acquisition of water versus phosphorus (Ho et al. 2005). Increasing atmospheric CO2 may call for changes in stomatal behavior or in nitrogen allocation to chlorophyll versus rubisco.
A particularly important class of tradeoff was identified by Colin Donald (1968). He hypothesized that there can be negative relationships between the ‘competitive ability of cultivars… and their capacity for yield in pure culture’. He described an ‘ideotype’ design for wheat plants with low competitiveness against neighbors – given good weed control, these would mostly be fellow wheat plants – but high community productivity. Consistent with Donald's ideas, past increases in crop yield potential have often been linked to decreased competitiveness, where competitiveness was in conflict with pure-culture yield (Reynolds et al. 1994).
The best-known example is the development of dwarf wheat and rice. Natural selection, driven by competition among plants, led to taller plants than was optimal for total seed production by a plant community. Greater investment in stems increased height and individual competitiveness for light, at the expense of collective production of seeds. Humans reversed past natural selection and selected for shorter, higher-yielding genotypes. When genotypes were grown separately, shorter rice plants that allocated more resources to grain had higher yield than taller ones that allocated more resources to stems. In competition, however, the shorter genotype rapidly disappeared (Jennings and de Jesus 1968).
Moving beyond stem height, Donald (1968) suggested many other improvements linked to competitiveness-versus-yield tradeoffs. Although even plant breeders who use the term ‘ideotype’ have not necessarily emphasized such tradeoffs (Rasmusson 1987), genetic increases in yield potential over decades have often coincided with Donald's suggestions. For example, higher-yielding wheat cultivars tend to have fewer stems per plant (Austin et al. 1980), as Donald proposed.
Here, we extend Donald's (1968) ideas regarding the benefits of erect leaves. When the sun is overhead, crops with more-erect leaves spread the available sunlight over more leaf area. Because photosynthesis tends to light-saturation, spreading the same amount of light over more leaf area can result in higher overall canopy photosynthesis. Manipulation of leaf angle in soybean (Kokubun 1988) and even in maize (Pendleton et al. 1968) showed a yield benefit from erect leaves, even though light-saturation is less likely in C4 maize. Interactions with nitrogen storage in leaves may also be important (Sinclair and Sheehy 1999). Trenbath and Angus (1975) found that many existing wheat cultivars had the ‘least favorable pattern of leaf inclination’, which they attributed to ‘earlier natural selection for aggressiveness’. Horizontal leaves may use light less efficiently, but they can shade neighboring competitors. Indeed, erect-leaf cultivars are usually less competitive with weeds (Tanner et al. 1966).
We hypothesized that solar tracking, or diaheliotropism, in which leaves turn to face the sun (Bonnet 1754), might have similar effects on canopy photosynthesis and competitiveness, throughout the day, as horizontal leaves do when the sun is overhead. Ehleringer and Forseth (1980) suggested that, if increased heat load is not a problem, then tracking in sparse canopies (e.g. isolated seedlings) would increase net photosynthesis by increasing total light interception. In dense canopies, however, they noted that solar tracking by upper leaves would reduce light available to lower leaves.
We evaluated the effects of solar tracking on canopy photosynthesis, using an experiment and a computer model. The ideal comparison would be between two genotypes differing only in tracking, but these were not available. We therefore simulated this comparison, using rotation in the horizontal plane to temporarily change leaf orientation. We then compared light interception and net canopy CO2 exchange in normal tracking mode to that with tracking disrupted. We argue below that this approach will overestimate the benefits of tracking, partly due to edge effects. Therefore, we also simulated the effects of tracking, without edge effects, using a computer model.
The effects of tracking on canopy photosynthesis were expected to depend on how sunlight and nitrogen are distributed with depth in the canopy. If tracking reduces light reaching lower leaves, then those leaves may lose more of their photosynthetic capacity (Hodgkinson 1974), perhaps by transferring nitrogen or other resources to the upper leaves, than if they were shaded less (Mooney and Gulmon 1982). Both the experiments and the model attempted to address possible longer-term effects of tracking on differences in leaf photosynthetic capacity with depth, in addition to the immediate effects of tracking on canopy photosynthesis.
Materials and methods
Experiment
We measured the effects of solar tracking on light interception and net canopy photosynthesis in sparse and dense canopies of alfalfa, Medicago sativa cv. ‘Weevlchek’. Plants were grown outdoors in tightly packed 8 × 8 arrays of 15-cm diameter pots in Beckley, West Virginia. Plants were watered daily, fertilized weekly with a nutrient solution, and cut back to 5 cm height at intervals of 15–32 days, as needed to prevent lodging.
To test the effects of solar tracking during growth on maintenance of photosynthetic capacity in lower leaves, one 8 × 8 array of pots was turned twice daily during weeks of growth, to reduce the effects of tracking on shading (and photosynthetic capacity) of lower leaves. A control group was never turned, but was allowed to track normally each day, presumably shading lower leaves more than in the disrupted (turned-during-growth) treatment.
Canopy photosynthesis and light interception by plants from both treatments were measured separately, as weather allowed, using a flow-through chamber fitted with a clear acrylic top (Fig. 1). Measurements were collected from a constructed alfalfa community consisting of 40 pots in tight hexagonal packing, approximately 0.8 m2 in total area, Pots, taken preferentially but not exclusively from inner rows of the 8 × 8 continuous-tracking or turned-daily growth arrays to reduce edge effects, were placed in the chamber in the same orientation as during growth. CO2 concentrations at the chamber output and input (supplemented so that concentration inside the chamber approximated ambient CO2) were measured with an infrared gas analyzer. Photosynthetically active radiation (PAR) beneath the canopy was measured with a line quantum sensor at soil surface height and compared with ambient PAR measured simultaneously with a spot quantum sensor outside the chamber. A slurry of water and ice in the lower, opaque, double wall of the chamber prevented condensation on the transparent top and maintained constant (±1°C) chamber air temperature within an experiment. All measurements were made on cloudless mornings, as diffuse light during cloudy weather would reduce the effects of solar tracking. This limited the total number of measurements that could be made during the summer. Solar elevation angles midway through experiments ranged from 40 to 44°. Leaf area index [LAI, ratio of leaf area to land area (Watson 1958)] was estimated by destructive sampling of leaf area from three randomly selected pots, using a leaf-area meter, after each experiment.
Figure 1.

Closing the lid on the photosynthesis chamber. Condensation is visible on ice-cooled lower section.
About 45 min after closing the chamber, and then twice at 15-min intervals, the table supporting the plants in the chamber was rotated 180° in the horizontal plane. Net photosynthesis rate was calculated as the difference in CO2 concentration between inlet and outlet air, times the air flow rate through the chamber, divided by the total area occupied by the pots. To avoid confounding solar-tracking effects with turning-induced transients (see below), data for each 15-min period at a given orientation were fitted to the equation P(t) = a+b·t−exp(−t/τ), where P(t) is the CO2 concentration difference (input minus outlet, proportional to photosynthesis rate) at time t, and a, b, and τ are constants. Steady-state photosynthesis was calculated from the linear term at the end of each 15-min turn. The effect of solar tracking on net canopy photosynthesis was calculated as a ratio: the average of the two steady-state values in the tracking orientation divided by steady-state photosynthesis at the intervening turned orientation [Applying this procedure to a ‘nontracking control’ (half of plants placed in chamber in reversed orientation) gave an original:turned ratio of 1.004 ± 0.012; mean ± SD, n = 3]. The relationship between LAI and tracking effects on canopy photosynthesis (tracking:turned ratio) was determined by linear and second-order-polynomial regression.
Computer model
We used a published mechanistic computer simulation model, ALFALFA (Denison and Loomis 1988), to simulate photosynthesis and light interception in the turning experiments described above, plus full-season simulations to predict the effect of solar tracking on annual forage production under field conditions. The model simulates growth of a field of identical alfalfa plants, simulating light interception, photosynthesis, transpiration, respiration, and growth of leaves, stems, and roots, with a 1-h time-step. FORTRAN source code is available from the author in electronic form, as is a 73-page user manual which also includes the source code (37 pages). It is not possible to prove that such a complex model is even approximately accurate under all conditions, but testing during model validation found that ten of twelve harvest yields at two locations (ranging from 1.5 to 6 t/ha) were predicted within 10%, while seasonal yields in an irrigation experiment at a third location (ranging from 6 to 22 t/ha) were predicted with r2 = 0.99 (Denison and Loomis 1988).
For the present paper, the light-interception and photosynthesis subroutine is particularly important, as only its predictions were compared to our measurements. This subroutine, based on the model of Duncan et al. (1967) simulates a multilayer canopy, with the number of layers and total leaf area increasing as the simulated crop grows taller. The fraction of solar radiation penetrating through each layer (i.e. not absorbed by leaves) was calculated from the cosine of incidence for the solar beam on leaves, cos(a), and the solar elevation, h, as e−LAI cos(a)/sin(h) (Duncan et al. 1967), Light intercepted within a layer was assumed to be divided equally over leaf area in that layer. The nonlinear response of photosynthesis to the absorbed light was based on published data for alfalfa (Sheehy et al. 1979). The model also includes temperature and water-stress effects on photosynthesis, but these would not affect the relative photosynthesis in tracking and disrupted orientations.
To compare model output with our experiments, we first generated virtual alfalfa canopies with a range of LAI values, by letting the model simulate growth until each target LAI was reached. We then restarted the model using the state variables (stem height, size of individual leaves, etc.) corresponding to each target LAI and simulated net canopy photosynthesis and light interception using solar elevations similar to that in the experiments, with cos(a) = 0.5 or 0.6 for nontracking and tracking orientations, respectively. These values were chosen to match overall canopy light penetration to actual measurements. To simulate the effects of light distribution in the canopy affecting leaf photosynthetic capacity, potential (light-saturated) photosynthesis was assumed to decrease linearly with physiological age (to zero at day 35), reducing the simulated photosynthesis of lower leaves. As a best-case alternative, we also ran the simulations with no decrease in photosynthetic capacity with leaf age.
Full-season simulations to estimate the effects of tracking on seasonal forage yield used the same cultivar- and location-specific parameters used in model validation (Denison and Loomis 1988), except that leaves in all layers were assumed to maintain cos(a) of either 0.6 (tracking) or 0.5 (nontracking), as explained above. Six cuttings were simulated, which is typical for the California locations where the model was developed and validated.
Results
Disrupting solar tracking, by turning plants 180° out of their normal tracking orientation, usually increased light penetration through the canopy, as exemplified by Fig. 2A for a canopy with LAI of 6.3. Of 15 experiments, only the one at the lowest LAI failed to show increased light penetration with disruption of solar tracking and tracking effects increased with LAI (Fig. 3A). Light-penetration results were similar for plants that had or had not been turned twice daily during growth (filled vs. open circles in Fig. 3A). Turning alfalfa 180° (either to or from the original tracking orientation) resulted in a transient decrease in photosynthesis. This phenomenon is shown in Fig. 2B, along with the fitted curves used to calculate steady-state photosynthesis. Shading half of the chamber with an opaque cover, then moving the cover to shade the other half, gave qualitatively similar transient decreases in photosynthesis (data not shown). This phenomenon may result from an induction requirement for photosynthesis in shaded leaves moving into sunflecks (Pearcy et al. 1985).
Figure 2.
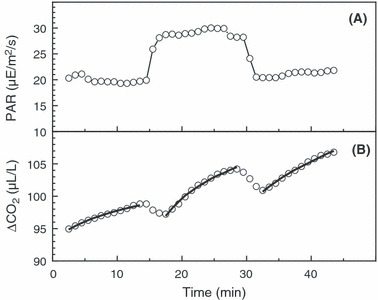
(A) Photosynthetically active radiation reaching the soil surface beneath solar-tracking alfalfa plants, with solar-tracking disrupted by 180° rotation from minutes 15–30. LAI of canopy was equal to 6.3. (B) Net CO2 uptake for the same plant community, with fitted curves used to estimate steady-state photosynthesis at each orientation.
Figure 3.
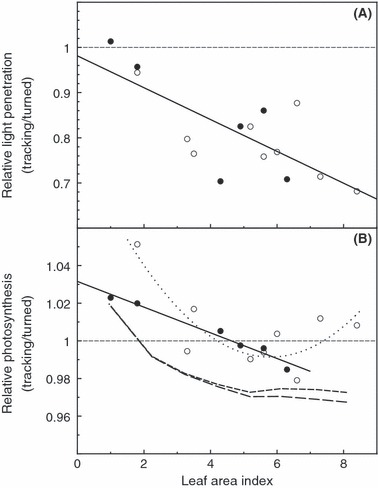
(A) Effects of tracking (ratio of tracking:turned) on light reaching soil surface below constructed canopies of alfalfa plants differing in LAI (leaf-area index; ratio of leaf area to ground area). Plants had been grown for weeks with (solid circles) or without (open circles) twice-daily turning to reduce shading of lower leaves by solar-tracking upper leaves during growth. Solid line indicates linear regression (P < 0.01, r2 = 0.56). (B) Effects of solar tracking on net canopy photosynthesis (ratio of tracking:turned). Symbols refer to pretreatments during weeks of growth, as for (A). Lines are regression for plants that had been turned during growth (solid) or not turned during growth (dotted), and model predictions, with (short dash) and without (long dash) decreased leaf photosynthetic capacity with age.
The effects of tracking on canopy photosynthesis varied with LAI (Fig. 3B). Each data point in Fig. 3B is the ratio of photosynthesis in the natural tracking orientation, divided by photosynthesis of the same plants in the turned (tracking-disrupted) orientation, with each ratio calculated from data similar to Fig. 2B. Different symbols indicate pretreatments imposed during prior weeks of growth. Open circles show tracking:turned photosynthesis ratios for plants that were never turned during growth. Closed circles show tracking:turned photosynthesis ratios for plants that had been turned twice per day, over weeks of growth, to disrupt solar tracking and allow the greater illumination of lower leaves expected in a hypothetical nontracking cultivar.
The most important results were qualitatively similar for plants from both pretreatments (turned or not turned during growth): direct effects of tracking on photosynthesis were small but positive for sparse (low-LAI) canopies, becoming small but negative as LAI increased (Fig. 3B). For plants turned during growth (filled circles), the second-order-polynomial term was not significant. The linear regression shown was highly significant (P < 0.001) with r2 = 0.97. The maximum canopy-photosynthesis benefit for solar tracking (extrapolated to LAI = 0) was about 3% and tracking reduced canopy photosynthesis above LAI = 4.5. For plants not turned during growth, there was considerably more scatter, so that two measurements with similar LAI often differed greatly. The second-order term in the regression shown was statistically significant (P < 0.05) with r2 = 0.71.
Model predictions of tracking effects on canopy photosynthesis (Fig. 3B) generally showed a greater photosynthetic cost of tracking at high LAI than was found experimentally. This was particularly true when it was assumed (lower, long-dash line) that lower leaves lost none of their original photosynthetic capacity with age and depth in the canopy. In the season-long simulation, the model predicted about 5% higher seasonal forage yield, 27 712 kg/ha, when we assumed less tracking, cos(a) = 0.5, relative to 26 468 kg/ha with the greater tracking, cos(a) = 0.6, more representative of existing cultivars.
Discussion
The discussion of experimental results will focus on plants that were turned twice daily during growth, because their relationship between LAI and tracking effects on photosynthesis was more consistent than for plants not turned during growth (Fig. 3B). Plants not turned during growth may have had increased pot to pot variability in the photosynthetic capacity of lower leaves due to greater light penetration along the edge of the synthetic canopy relative to pots in the interior of the canopy (edge effects) as the plants grew undisturbed. By temporarily disrupting solar tracking, twice-daily turning during growth increased light penetration deeper into the canopy, which presumably helped to maintain photosynthetic capacity in lower leaves and reduced pot to pot variability in this characteristic. Since some photosynthesis measurements included more edge-grown plants than others, the measured photosynthetic opportunity cost of tracking was more variable in experiments using plants that were not turned during growth. In the two highest-LAI photosynthesis measurements (Fig. 3B), shading during growth in the not-turned pretreatment may have reduced the photosynthetic capacity of lower leaves well below what it would be in a nontracking cultivar, eliminating some potential photosynthetic benefit of disrupting solar tracking.
Under our well-watered conditions, solar tracking in alfalfa was found to increase light interception, relative to the disrupted leaf orientation achieved by turning in the horizontal plane. This would not necessarily be true for plants under drought conditions (Berg and Hsiao 1986). Diaheliotropism increased net canopy photosynthesis at low LAI in our experiment, but only by a few percent. For LAI around 1, model and experiment agreed on a photosynthetic benefit of about 2% from tracking (Fig. 3B).
Model and experiment also agreed that this benefit decreased with increasing LAI, but there were quantitative discrepancies between model and experiment at high LAI. When a model disagrees with reality, the model is clearly wrong. In this case, however, our artificial community of alfalfa plants in pots is itself a model system. Edge effects would be much stronger in our experiments than in the field. In contrast to the field situation, a significant fraction of the light reaching our lower leaves came from the side, rather than down through the canopy. This extra light means that lower leaves would experience less tracking-induced-shading cost than if they were completely surrounded by other plants. It is therefore likely that, at high LAI, the experiments somewhat underestimated the photosynthetic cost of solar tracking. It is possible, therefore that the computer model gave a more accurate picture of tracking effects than the experiments with the model plant community. This hypothesis could be tested using even smaller model plant communities (20 pots rather than 40, say), which would be predicted to give similar results at low LAI, but with little or no cost of tracking at high LAI.
The whole-season simulations suggested a 5% photosynthetic cost to solar tracking. In these simulations, benefits at low LAI were outweighed by costs at high LAI, even with six cuttings per year. Alfalfa cut fewer times per year could spend less time in the low-LAI state typical of early regrowth, so the net photosynthetic cost of solar tracking could be even greater. Together, model and experiment show that the photosynthetic benefits of solar tracking are low for alfalfa and that tracking at high LAI is more likely to reduce canopy photosynthesis than to increase it.
Heliotropism is a complex behavior dependent on many genes, so loss-of-function mutations that eliminate tracking must have arisen repeatedly over the course of evolution. The evolutionary persistence of solar tracking, despite its apparent photosynthetic cost at high LAI, therefore requires explanation. One possibility is that photosynthetic benefits of tracking by seedlings or other low-LAI (e.g. recently grazed) plants are sufficient to maintain diaheliotropism, despite its photosynthetic costs once plants grow larger and start shading their own lower leaves. This would be analogous to antagonistic pleiotropy maintaining alleles for early reproduction at the expense of longevity (Williams 1957). Travis and Reed (1983) reported that cos(a) changes with depth in the canopy, but unfortunately the equation they used to calculate cos(a) from their leaf-angle data is incorrect (Comstock and Mahall 1985).
An alternative hypothesis is that solar tracking even in dense canopies makes positive contributions to lifetime fitness. At LAI = 5, a diaheliotropic plant might reduce light available to seedlings growing in its shade to 80% of that below a nontracking plant (Fig. 3A), reducing their photosynthesis nearly 20%, while reducing its own photosynthesis by less than 1% (Fig. 3B). Sacrificing any fitness to injure another member of the same species would be an example of spite (Hamilton 1970). However, a perennial plant may receive direct, albeit delayed, fitness benefits from suppressing potential competitors, in which case solar tracking would not be an example of spite. Furthermore, the shaded competitors could often be members of other species, even in agriculture.
Our experiments used monospecific stands of alfalfa, but real alfalfa fields usually include weeds. Alfalfa plants that track the sun are more competitive against fellow alfalfa plants, which may reduce potential yield by 5% or more, but tracking plants are also more competitive against weeds. A nontracking alfalfa cultivar, or one with reduced tracking at high LAI, might be useful only in fields with excellent weed control.
Literature cited
- Austin RB, Bingham J, Blackwell RD, Evans LT, Ford MA, Morgan CL, Taylor M. Genetic improvements in winter wheat yields since 1900 and associated physiological changes. Journal of Agricultural Science, Cambridge. 1980;94:675–689. [Google Scholar]
- Berg VS, Hsiao TC. Solar tracking: light avoidance induced by water stress in leaves of kidney bean seedlings in the field. Crop Science. 1986;26:980–986. [Google Scholar]
- Bonnet C. Recherches sur l'usage des feuilles dans les plantes, et sur quelques autres sujets relatifs a l'histoire de la vegetation. Chez Elje Luzac, Gottingen. 1754 [Google Scholar]
- Comstock JP, Mahall BE. Drought and changes in leaf orientation for two California chaparral shrubs: Ceanothus megacarpus and Ceanothus crassifolius. Oecologia. 1985;65:531–535. doi: 10.1007/BF00379668. [DOI] [PubMed] [Google Scholar]
- Denison RF. Darwinian agriculture: real, imaginary and complex tradeoffs as constraints and opportunities. In: Sadras VO, Calderini DF, editors. Crop Physiology: Applications for Genetic Improvement and Agronomy. Amsterdam: Elsevier; 2009. pp. 215–234. [Google Scholar]
- Denison RF, Loomis RS. An Integrative Physiological Model of Alfalfa Growth and Development. Oakland: University of California; 1988. [Google Scholar]
- Denison RF, Kiers ET, West SA. Darwinian agriculture: when can humans find solutions beyond the reach of natural selection? Quarterly Review of Biology. 2003;78:145–168. doi: 10.1086/374951. [DOI] [PubMed] [Google Scholar]
- Donald CM. The breeding of crop ideotypes. Euphytica. 1968;17:385–403. [Google Scholar]
- Duncan WG, Loomis RA, Williams WA, Hanau R. A model for simulating photosynthesis in plant communities. Hilgardia. 1967;38:181–205. [Google Scholar]
- Ehleringer JR, Forseth IN. Solar tracking by plants. Science. 1980;210:1094–1098. doi: 10.1126/science.210.4474.1094. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Selfish and spiteful behaviour in an evolutionary model. Nature. 1970;228:1218–1220. doi: 10.1038/2281218a0. [DOI] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology. 2005;32:737–748. doi: 10.1071/FP05043. [DOI] [PubMed] [Google Scholar]
- Hodgkinson KC. Influence of partial defoliation on photosynthesis, photorespiration and transpiration by lucerne leaves of different ages. Australian Journal of Plant Physiology. 1974;1:561–578. [Google Scholar]
- Jennings PR, De Jesus J. Studies on competition in rice. I. Competition in mixtures of varieties. Evolution. 1968;22:119–124. doi: 10.1111/j.1558-5646.1968.tb03455.x. [DOI] [PubMed] [Google Scholar]
- Kellogg EA. Phylogenetic aspects of the evolution of C4 photosynthesis. In: Sage RF, Monson RK, editors. C4 Plant Biology. San Diego: Academic Press; 1999. pp. 411–444. [Google Scholar]
- Kokubun M. Design and examination of soybean ideotypes. Japan Agricultural Research Quarterly. 1988;21:237–243. [Google Scholar]
- Mooney HA, Gulmon SL. Constraints on leaf structure and function in relation to herbivory. Bioscience. 1982;32:198–206. [Google Scholar]
- Nelson DE, Repetti PP, Adams TR, et al. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16450–16455. doi: 10.1073/pnas.0707193104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearcy RW, Osteryoung K, Calkin HW. Photosynthetic responses to dynamic light environments by Hawaiian trees. Time course of CO2 uptake and carbon gain during sunflecks. Plant Physiology. 1985;79:896–902. doi: 10.1104/pp.79.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton JW, Smith GE, Winter SR, Johnston TJ. Field investigations of the relationships of leaf angle in corn (Zea mays L.) to grain yield and apparent photosynthesis. Agronomy Journal. 1968;60:422–424. [Google Scholar]
- Rasmusson DC. An evaluation of ideotype breeding. Crop Science. 1987;27:1140–1146. [Google Scholar]
- Reynolds MP, Acevedo E, Sayre KD, Fischer RA. Yield potential in modern wheat varieties: its association with a less competitive ideotype. Field Crops Research. 1994;37:149–160. [Google Scholar]
- Sheehy JE, Woodward FI, Jones MB, Windram A. Microclimate, photosynthesis and growth of lucerne (Medicago sativa L.). I. Microclimate and photosynthesis. Annals of Botany. 1979;44:693–707. [Google Scholar]
- Sinclair TR, Sheehy JE. Erect leaves and photosynthesis of rice. Science. 1999;283:1456–1457. [Google Scholar]
- Tanner JW, Gardener CJ, Stoskopf NC, Reinbergs E. Some observations on upright-leaf-type small grains. Canadian Journal of Plant Science. 1966;46:690. [Google Scholar]
- Travis RL, Reed R. The solar tracking pattern in a closed alfalfa canopy. Crop Science. 1983;23:664–668. [Google Scholar]
- Trenbath BR, Angus JF. Leaf inclination and crop production. Field Crop Abstracts. 1975;28:231–244. [Google Scholar]
- Watson DJ. The dependence of net assimilation rate on leaf-area index. Annals of Botany. 1958;22:37–54. [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]


