Abstract
Gene flow from transgenic crops allows novel traits to spread to sexually compatible weeds. Traits such as resistance to insects may enhance the fitness of weeds, but few studies have tested for these effects under natural field conditions. We created F2 and F3 crop–weed hybrid lineages of genetically engineered rice (Oryza sativa) using lines with two transgene constructs, cowpea trypsin inhibitor (CpTI) and a Bt transgene linked to CpTI (Bt/CpTI). Experiments conducted in Fuzhou, China, demonstrated that CpTI alone did not significantly affect fecundity, although it reduced herbivory. In contrast, under certain conditions, Bt/CpTI conferred up to 79% less insect damage and 47% greater fecundity relative to nontransgenic controls, and a 44% increase in fecundity relative to the weedy parent. A small fitness cost was detected in F3 progeny with Bt/CpTI when grown under low insect pressure and direct competition with transgene-negative controls. We conclude that Bt/CpTI transgenes may introgress into co-occurring weedy rice populations and contribute to greater seed production when target insects are abundant. However, the net fitness benefits that are associated with Bt/CpTI could be ephemeral if insect pressure is lacking, for example, because of widespread planting of Bt cultivars that suppress target insect populations.
Keywords: biosafety, biotechnology, competition, genetically engineered, introgression, red rice, risk assessment, weediness
Introduction
The environmental release of transgenic crops has generated considerable debate about the ecological and evolutionary consequences of adopting these crops. A major biosafety concern relates to unwanted effects because of transgene flow from genetically engineered (GE) crops to their wild or weedy relatives (Ellstrand 2003; Snow et al. 2005). For example, the acquisition of traits such as resistance to herbicides, insects, and diseases might allow wild/weedy relatives to become more abundant, perhaps exacerbating weed management problems and displacing non-GE wild genotypes in some situations (Lu and Snow 2005; Andow and Zwahlen 2006).
Crop-to-wild gene flow is well documented in many species (Ellstrand 2003), as is the stable inheritance, expression, and efficacy of transgenes in crop–wild hybrids (Zhu et al. 2004; Ammitzbøll et al. 2005; Xia et al. 2009). Novel transgenic traits that enhance fitness are expected to introgress into recipient populations, whereas traits that are associated with fitness costs may eventually be lost (Jenczewski et al. 2003). However, studies of such fitness consequences under natural biotic and abiotic conditions are uncommon, in part because so few of the currently grown transgenic crops can hybridize with feral, weedy, or wild relatives (exceptions include canola and squash). Nonetheless, the number and diversity of transgenic crops, including the introduction of relatively undomesticated biofuel crops, is expected to increase dramatically in the coming decade (Gressel 2008).
Crop traits such as herbicide resistance are clearly advantageous to weeds that are exposed to these herbicides (Hall et al. 2000). In contrast, possible benefits of transgenes for resistance to diseases or insect herbivores are less obvious and depend on whether wild/weedy populations typically are limited by these biotic factors. Transgenes for virus resistance can be highly advantageous to wild squash in North America (Laughlin et al. 2009; Sasu et al. 2009) and weedy clover populations in Australia (Godfree et al. 2007). In sunflower, field experiments to test for fitness benefits of a Bt transgene showed that transgenic crop–wild hybrids produced significantly more seeds per plant under natural levels of herbivory, with no apparent fitness costs (Snow et al. 2003). In another study of sunflowers, no benefit of a transgene for white mold resistance was found in artificially infected crop–wild hybrids (Burke and Rieseberg 2003).
Here, we investigated the effects of two transgenes for insect resistance in rice, cowpea trypsin inhibitor (CpTI) and a linked Bt/CpTI construct, on the fecundity of hybrids progeny between cultivated and weedy rice. Rice (Oryza sativa L.) is a staple food for nearly half of the global population (FAO 2004). In 2005, Iran became the first country to commercialize Bt transgenic rice (James 2006). China has invested heavily in developing disease- and insect-resistant rice varieties, many of which appear to be close to approval for environmental release. The government of China approved biosafety certificates for Bt rice in 2009 (James 2009), and locally bred rice varieties with Bt transgenes could be widely grown within the next few years. In the USA, hundreds of GE rice lines have been tested in precommercial field trials, but only one type of herbicide-tolerant rice has been deregulated to date and it has yet to be widely grown [Information Systems for Biotechnology, http://www.isb.vt.edu/; (USDA 2006)]. Pharmaceutical-producing GE rice is grown in the USA under a permit system with bioconfinement requirements and small-scale production (National Research Council 2002).
Transgenes from cultivated rice are expected to spread to weedy rice (O. sativa f. spontanea), also known as red rice, which is a noxious weed that occurs in rice fields in many regions worldwide (Delouche et al. 2007; Londo and Schaal 2007). Weedy rice belongs to the same species as cultivated rice (Harlan and Wet 1971) and possesses variable levels of seed shattering, seed dormancy, chilling tolerance, presence of awns, and a red pericarp (Oard et al. 2000; Gealy et al. 2006). Rice fields are the primary habitat for weedy rice, and the weed can substantially reduce crop yields because it competes for light, space, nutrients, and water and cannot be harvested for food (Delouche et al. 2007). Also, the adventitious presence of dark-colored weedy rice grains can reduce the market value of cultivated rice (Arrieta-Espinoza et al. 2005).
Although cultivated and weedy rice are primarily self-pollinating, gene flow from crop-to-weed and weed-to-crop is well documented (Zhang et al. 2003; Chen et al. 2004; Messeguer et al. 2004; Shivrain et al. 2009). Therefore, it is widely assumed that transgenes introduced into modern rice cultivars will make their way into weedy rice populations (Gealy et al. 2003). Given that gene flow from cultivated rice to co-occurring weedy rice is unavoidable, it is important to understand the consequences of transgene introgression into wild/weedy rice populations. For example, many authors have warned that transgenes for herbicide resistance are likely to spread rapidly to weedy rice unless strong mitigation procedures are in place (Olofsdotter et al. 2000; Kumar et al. 2008; Gressel and Valverde 2009). In Vietnam, Cohen et al. (2008) showed that weedy rice often flowered simultaneously with cultivated rice within the same fields and shared many of the crop's insect pests and pathogens. This suggests that transgenes for insect and disease resistance could introgress into weedy rice and provide protection.
Our previous studies showed that Bt and Bt/CpTI transgenes can substantially enhance crop yields when target insects are abundant, while no beneficial effects on fecundity were seen for CpTI alone (Table 1; Appendix 1). Under very low insect pressure, when net fitness costs might be detected, we observed significant yield reductions because of the Bt/CpTI transgene in cultivated rice in two of the three years (Chen et al. 2006; Xia et al. 2010; Table 1; Appendix 1). However, these costs were seen only when the transgenic plants were grown intermixed with nontransgenic controls, which is likely to amplify small differences in competitive ability. We also found that F1 hybrids between the GE lines and three weedy rice strains had generally lower seed production per plant, but higher seed germination and survival than their weedy parents (Cao et al. 2009). Thus, the F1 generation could constitute an effective genetic bridge for transgenes to be transmitted to subsequent generations of weedy rice in this system.
Table 1.
Summary of experimental procedures, insect pressure, and effects of transgenes on fecundity in cultivated rice and crop–weed hybrid progeny
| Experimental procedure | Insect pressure | Difference in no. of seeds per transgenic relative to nontransgenic plant under pure or mixed cultivation† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year* | Plant type | Pot versus plot | No. of replicates | No. of plants sampled/replicate (mixed, pure cultivation) | Percentage of damage on non-genetically engineered plants | Bt pure | Bt mixed | Bt/CpTI pure | Bt/CpTI mixed | CpTI pure | CpTI mixed |
| 2003 | Crop | Pot | 20 | 6, 6 | 4 | 15% | −10% | 19% | −30% | −4% | −15% |
| 2003 | Crop | Pot | 20 | 6, 6 | 30 | 36% | 65% | 61% | −9% | 21% | 4% |
| 2004 | Crop | Plot | 5 | 30, 60 | 1 | −15% | −52% | 24% | −53% | 26% | −28% |
| 2004 | Crop | Plot | 5 | 30, 60 | 14 | −4% | −2% | 0% | 3% | −6% | −3% |
| 2006 | Crop | Plot | 3 | 42, 63 | 1 | 3% | −4% | 5% | −42% | 2% | −28% |
| 2006 | Crop | Plot | 3 | 42, 63 | 23 | 19% | 12% | 45% | 33% | 41% | −8% |
| 2008 | Weedy F2 hybrid | Plot | 8 | 18§, 36 | 8‡ | – | – | −6% | 11%‡ | −2% | −2%§ |
| 2008 | Weedy F2hybrid | Plot | 8 | 18§, 36 | 28‡ | – | – | 25% | 34%‡ | 6% | −4%§ |
| 2009 | Weedy F3 hybrid | Plot | 4 | 18, 36 | 8‡ | – | – | −3% | −19% | −4% | 13% |
| 2009 | Weedy F3hybrid | Plot | 4 | 18, 36 | 22‡ | – | – | 47% | 3% | 9% | 10% |
Data from the present study were collected in 2008–2009, and data from experiments in 2003–2006 were from Chen et al. (2006) and Xia et al. (2010). Boldfaced values with gray shade indicate natural insect pressure, while unshaded values with normal face indicate low insect pressure.
Differences was estimated by the percent increase (fitness benefit)/decrease (fitness cost) in fecundity of transgenic rice or crop–weed progeny relative to nontransgenic controls; the values with underlines indicate significance at P < 0.05.
Calculated based on the average of the nontransgenic control of cowpea trypsin inhibitor (CpTI) and Bt/CpTI.
In 2008, the mixed treatment involved crop plants as competitors. In all other years, the mixed treatment involved transgenic and nontransgenic plants competing against each other in the same plot or pot.
The goals of the present study were to determine the effects of the CpTI and Bt/CpTI transgenes on herbivory and fecundity in the F2 and F3 hybrid progeny under two levels of insect pressure (low versus natural). To address these questions, we used segregating transgene-positive and transgene-negative lineages generated from both transgenic constructs under monotypic versus mixed competition treatments. We also compared the fecundity of F3 hybrid progeny with that of their weedy parents to evaluate the potential for GE weedy rice to become more abundant than its predecessor over time. Information about the relative performance of transgenic weedy rice originating from crop–weed hybridization is essential for determining whether gene flow from current or future GE rice lines could have significant adverse unintended consequences.
Materials and methods
Cultivar and weedy parents for crosses
Two GE rice lines produced by Fujian Academy of Agricultural Sciences, China, were used as pollen donors to create the crop–wild hybrid lineages (Xia et al. 2010). These agrobacterium-transformed lines were bred beyond the seventh generation from an inbred traditional Minghui-86 variety. One line (CpTI) has a CpTI (cowpea trypsin inhibitor) gene, while the other (Bt/CpTI) has the CpTI gene linked to a Bt cryIAc (Bacillus thuringiensis) gene in a double insertion. A selectable marker gene (hy, for hygromycin resistance) is tightly linked to each transgene. Bt, CpTI, and hy genes have constitutive promoters of ubiquitin (derived from maize), ActID (derived from rice), and CaMV35s, respectively. These insect-resistant GE lines were produced to deter lepidopteran pests such as rice stem borers (Scirpophaga incertulas, Chilo suppressalis, and Sesamia inferens) and rice leaf-folder (Cnaphalocrocis medinalis). Because only one transgenic event of each type was available to us, generalizations about other CpTI and Bt/CpTI events should be made with caution. For brevity, we refer to fitness-related differences between transgene-positive and transgene-negative plants as effects of the transgenes, with the caveat that some of these differences could be influenced by somaclonal variation, pleiotropy, or position effects, such as crop genes that are linked to the insertion sites. Transgenic lines that are being developed for commercial use in China are not expected to have the antibiotic resistance marker used in this study and they are derived from different insertion events. Although our previous studies included a Bt cultivar without the CpTI gene (Chen et al. 2006; Cao et al. 2009), owing to logistical problems, we were not able to obtain hybrid progeny from this line for the present study.
Two weedy rice strains Wa and Wb donated by Dr H. S. Suh of Yeungnam University from South Korea were available and used in this study. Strain Wa is short and has short, narrow leaves, red awns, and medium tillering ability, while strain Wb is taller and has longer, narrow leaves, no awns, and medium tillering ability. Differences between the two strains in fecundity and individual seed mass were negligible (Cao et al. 2009). Given the similar rice farming and climate conditions in South Korea and NE and E China, the results obtained from the two Korean weedy rice strains could represent the situation in rice-growing regions in NE and E China.
Crop–weed hybrid lineages
To compare fitness-related traits of hybrid progeny that differed in the presence or absence of transgenes, F2 and F3 hybrid progeny were generated by selfing F1 and F2 plants, respectively (Fig. 1). Because weedy rice is a predominantly self-pollinating taxon, F2 and F3 generations generated by selfing will be much more common than backcrossed generations. F1 hybrids were obtained from artificial crosses between the two weedy rice strains each including more than 20 individuals and two insect-resistant GE rice lines, resulting in four distinct hybrid lineages (Cao et al. 2009). Weedy rice strains were used as the maternal parents and the GE rice lines as the paternal parents in crosses (Fig. 1). Both weedy strains were used in the F2 hybrid progeny, but only Wa (chosen randomly) was used for the F3.
Figure 1.
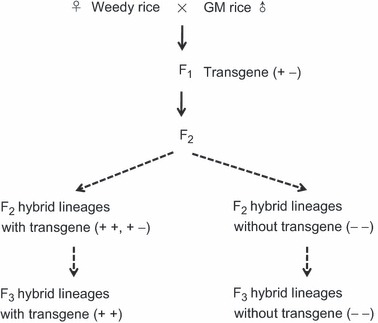
A pedigree illustrating the production of F2 and F3 crop–weed hybrid lineages in rice with insect-resistant transgenes (transgene-positive: + − or + +) or without the transgenes (transgene-negative: −−).
Hybrid progeny were identified for the presence or absence of transgenes. For identification of F2 individuals, total genomic DNA was extracted from young leaf samples from each F2 seedling beyond the 3-leaf stage, prior to transplanting, following the method described by Doyle and Doyle (1987). The identification of transgene-positive and transgene-negative seedlings involved PCR amplification of the target transgenes: the CpTI gene for individuals derived from the CpTI insect-resistant rice line, and the Bt gene for individuals from the Bt/CpTI insect-resistant rice line (Rong et al. 2005). For plants that scored positive for the transgene, we did not attempt to determine whether they were homozygous (++) or hemizygous (+−). In weedy rice, hemizygous and homozygous plants are expected to have similar levels of transgene expression, as shown in wild rice hybrids containing the Bt transgene (Xia et al. 2009), but this was not confirmed in the present study.
For F3 hybrid progeny, we used the hygromycin-B water solution treatment to identify transgenic individuals (Rong et al. 2005). Groups of about 15 seeds from each transgenic F2 plant were screened. F2 individuals from which all the 15 seedlings survived from the hygromycin-B treatment were identified as the transgenic homozygote (++), whereas those from which all the 15 seedlings died from the hygromycin-B treatment were identified as the nontransgenic homozygote (−−). F3 seeds from hemizygous F2 plants were not included in the F3 field experiment.
Design of field experiments
We evaluated insect damage and fitness-related traits of the F2 and F3 hybrid progeny, with or without transgenes, in 2008 and 2009, respectively. The field plots were located at designated Biosafety Assessment Centers in Fuzhou, Fujian Province, China, where the plants were exposed to naturally occurring levels of insect damage (no insecticides). To test for possible fitness costs of the transgenes, we also included a ‘low insect pressure’ treatment by spraying replicated plots with insecticides that are commonly used in rice fields (Methamidophos, Folimat, Buprofezin, and Monosultap).
The F2 progeny were evaluated in a randomized, factorial design experiment that included the following factors for each transgene (CpTI or Bt/CpTI): insect pressure (low versus natural), transgene (present [++, +−] versus absent [−−]), competition mode (monoculture versus mixture with cultivated rice), and weedy strain (Wa versus Wb), with four replicate plots for each treatment combination. Within each plot, 36 seedlings were transplanted in a 6 × 6 grid with 20 cm-spacing between seedlings, with one seedling in each hill. In the mixed competition plots to simulate the transplanting rice field, half of the plants were individuals of cultivated Minghui-86 (which was not sampled). Therefore, the numbers of experimental plants that were sampled in each plot (replicate) were 36 plants for monocultures, which we refer to as ‘pure’, and 18 plants for mixed competition plots, which we refer to as ‘mixed’. Data from these plants were averaged to provide one measurement for each of the four replicate plots for each treatment combination.
The F3 generation was evaluated in 2009 using similar procedures, with the following exceptions. Only the Wa weedy hybrid lineage was used, and four extra plots were added to include pure plantings of the Wa weedy parent. As noted earlier, all of the transgenic F3 plants were homozygous for each transgene. In 2009, the mixed competition treatment included 18 transgenic and 18 nontransgenic F3 plants from the same GE lineage in an alternating pattern, in an attempt to magnify any differences in their growth rates and fecundity (no cultivated Minghui-86 plants were used). In addition, plants in the pure plots were 20 cm apart, while those in the mixed competition plots were only 15 cm apart to intensify competition. Finally, each of the four replicate plots for the F3 generation included a border row of extra plants (same genotypes) that were not sampled.
Seedlings were started in a small plot at Fudan University, Shanghai, where no target insects were found. F2 seedlings were transplanted to the field site in Fuzhou 40 days after seed germination and F3 seedlings were transplanted 28 days after seed germination. There were no significant differences in seedling survival between lineages (unpublished data). Before transplanting, the field site had been treated with herbicide and weeded by hand. Fertilizer was applied at the tillering stage [∼1 to 1.2 kg nitrogen (urea) per 100 m2], as is common for rice cultivation in the Fuzhou area.
Data collection and analysis
Data collection methods were similar for both years of the study. We measured plant height, number of tillers per plant, the number of blasted tillers (by rice stem borers) and folded leaves (by leaf-folders) at the beginning of flowering time. To avoid seed loss by natural seed shattering, all panicles of an individual were enclosed in a nylon mesh bag 10 days after flowering. Panicles were collected for measurements of number of seeds per plant, seed set (percent of spikelets with filled seeds), and 1000-seed weight. To characterize insect damage, we recorded the percent of blasted tillers and folded leaves. An insect damage index was calculated as the sum of these two percents for each plant.
The four-way ANOVA analysis was carried out by the GLM multivariate procedure for the CpTI and Bt/CpTI hybrid lineages separately in F2 hybrid progeny. Because the factor of weedy strains (Wa versus Wb) did not show significant effect on fecundity (the number of good seeds), the main variable of interest, this factor was removed from the ANOVA analysis. Thus, for the F2 generation, we used a three-way ANOVA to test the effects of insect pressure (low versus natural), transgenic genotype (positive versus negative), and competition (pure versus mixed) on insect damage and number of seeds per plant. All the model factors were considered as fixed under the GLM multivariate procedure. To further examine transgene effects on insect damage and fecundity, independent t-tests (for F2 and pure plots in F3) and paired t-tests (for mixed plots in F3) were used to test for significant differences between means for transgene-positive and transgene-negative plants in each lineage and treatment combination. In addition, for fecundity and other fitness-related traits, we used Duncan's multiple range test followed by the more conservative Bonferroni correction to test for significant differences among means of four groups [CpTI (+)versus CpTI (−) versus Bt/CpTI (+) versus Bt/CpTI (−)] in the F2 generation, and five groups [CpTI (+) versus CpTI (−) versus Bt/CpTI (+) versus Bt/CpTI (−)versus Wa] in the F3 generation (pure competition only). Independent t-test (for F2 and pure plots in F3) and paired t-test (for mixed plots in F3) were used to test for significant differences between means for transgene-positive and transgene-negative plants in each lineage and treatment combination. All statistical analyses were performed using the software package SPSS ver. 15.0 for Windows (SPSS Inc., Chicago, IL, USA, 2006).
Results
Insect pressure and competition
Natural levels of insect damage on nontransgenic plants were relatively high in both years of the experiment (28 and 22%; Table 1; Figs 2 and 3). Insecticide applications substantially reduced insect damage (to ∼8% in both years). These differences caused by insecticides allowed us to test for net fitness benefits of the transgenes when plants were exposed to target herbivores, as well as net fitness costs of the transgenes when insect pressure was reduced (note that fitness costs can be offset by benefits and vice versa, resulting in no net fitness effects; e.g. Chen et al. 2006). Seed production was lower in the mixed competition plots than with pure competition in both generations. This treatment involved competition with the cultivar in 2008, and closer spacing between plants than with pure competition in 2009. Major effects of the transgenes on plant performance are presented in more detail below and in Tables 2 and 3. The transgenes had no effect on seedling survival (data not shown), which will not be considered further.
Figure 2.
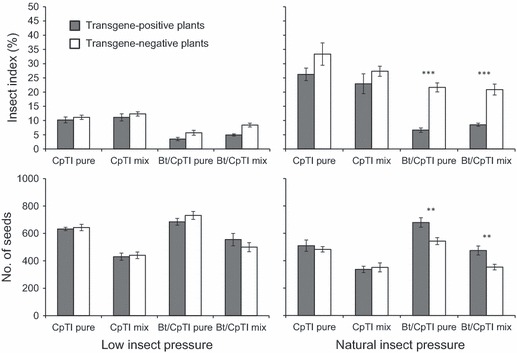
Effects of transgenic genotype (positive versus negative), insect pressure (low versus natural), and competition (pure versus mixed) on insect damage and number of seeds per plant in crop–weed hybrids from two transgene crop lines, cowpea trypsin inhibitor (CpTI) or Bt/CpTI, in the F2 generation. Data from two weedy strains, Wa and Wb, are pooled (N = 8); means and SE are shown. The comparison was made between transgene-positive and transgene-negative plots based on the independent t-tests. Levels of significance: **P < 0.01, ***P < 0.001.
Figure 3.
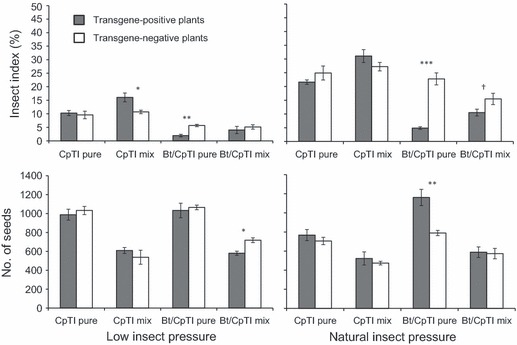
Effects of transgenic genotype (positive versus negative), insect pressure (low versus natural), and competition (pure versus mixed) on insect damage and number of seeds per plant in crop–weed hybrids from two transgenic crop lines, cowpea trypsin inhibitor (CpTI) or Bt/CpTI, in the F3 generation. Means and SE are shown. The comparison was made between transgene-positive and transgene-negative plots based on independent t-tests (for pure plots) and paired t-tests (mixed plot). Levels of significance: *P < 0.05, **P < 0.01, ***P < 0.001; †P < 0.10.
Table 2.
Effects of the two transgenic events (cowpea trypsin inhibitor (CpTI) and Bt/CpTI) on fitness-related traits in F2 progeny of crop–weed hybridization under low versus natural insect pressure and pure versus mixed cultivation
| Pure cultivation | Mixed cultivation | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | CpTI (+) | CpTI (−) | Bt/CpTI (+) | Bt/CpTI (−) | CpTI (+) | CpTI (−) | Bt/CpTI (+) | Bt/CpTI (−) |
| Low insect | ||||||||
| Plant height (cm) | 105.9 ± 2.3a | 104.8 ± 2.7a | 109.2 ± 0.2a | 109.1 ± 3.5a | 104.0 ± 2.5a | 105.4 ± 2.3a | 106.5 ± 2.5a | 105.6 ± 1.4a |
| No. of tillers | 22.0 ± 1.2a | 21.6 ± 1.3a | 24.0 ± 2.4a | 23.6 ± 2.1a | 16.1 ± 1.2a | 17.3 ± 1.2a | 17.2 ± 1.5a | 16.2 ± 1.2a |
| No. of panicles | 18.5 ± 0.8a | 18.4 ± 1.0a | 18.7 ± 0.8a | 18.8 ± 0.9a | 11.9 ± 0.5a | 12.4 ± 0.7a | 13.6 ± 1.2a | 12.2 ± 1.0a |
| No. of seeds | 632.1 ± 12.1a | 643.1 ± 23.1a | 684.9 ± 24.8ab | 731.3 ± 29.5b | 430.0 ± 26.3a | 440.1 ± 23.8a | 554.7 ± 45.5b | 499.6 ± 33.5ab |
| Seed set | 46.2 ± 1.3a | 48.4 ± 1.3ab | 47.2 ± 1.5ab | 50.6 ± 1.4b | 44.5 ± 2.7a | 44.4 ± 2.1a | 50.6 ± 1.5a | 48.6 ± 2.2a |
| 1000-seed weight (g) | 23.3 ± 0.4a | 22.6 ± 0.2ab | 22.3 ± 0.3b | 22.8 ± 0.3ab | 22.7 ± 0.6a | 22.5 ± 0.5a | 22.5 ± 0.4a | 22.6 ± 0.2a |
| Natural insect | ||||||||
| Plant height (cm) | 98.9 ± 2.0a | 96.1 ± 3.0a | 112.2 ± 2.3b | 107.7 ± 1.3b | 100.2 ± 1.1a | 102.1 ± 3.3a | 107.1 ± 2.8a | 102.0 ± 1.9a |
| No. of tillers | 19.9 ± 1.3a | 19.3 ± 1.4a | 22.8 ± 2.2a | 20.2 ± 1.8a | 15.6 ± 1.0a | 15.6 ± 0.8a | 16.5 ± 1.3a | 15.4 ± 1.2a |
| No. of panicles | 15.1 ± 0.4a | 15.4 ± 0.3a | 19.5 ± 0.7b | 16.2 ± 0.3a | 10.9 ± 0.8a | 11.3 ± 1.2a | 14.3 ± 0.7b | 12.6 ± 1.1ab |
| No. of seeds | 509.2 ± 41.4a | 482.5 ± 19.7a | 678.5 ± 34.5b | 542.6 ± 25.3a | 336.5 ± 23.4a | 350.5 ± 32.8a | 473.8 ± 32.7b | 352.9 ± 20.1a |
| Seed set | 41.4 ± 2.6a | 39.9 ± 2.5a | 48.6 ± 1.7b | 39.9 ± 2.0a | 42.2 ± 1.1ab | 44.3 ± 2.2a | 45.4 ± 1.7a | 37.6 ± 1.3b |
| 1000-seed weight (g) | 21.8 ± 0.3ab | 20.9 ± 0.3a | 22.2 ± 0.3b | 21.4 ± 0.5ab | 22.4 ± 0.5a | 22.1 ± 0.5a | 22.4 ± 0.3a | 21.3 ± 0.4a |
Different letters after the means and standard error (±) in the same row indicate significant differences according to Duncan's multiple range tests followed by Bonferroni correction in the same treatment. Data from the two weedy strains were combined (see text). N = 8 plots.
Table 3.
Effects of the two transgenic events on fitness-related traits in F3 progeny of crop–weed hybridization compared with the weedy parent (Wa) under low versus natural insect pressure in pure cultivation
| Pure cultivation | |||||
|---|---|---|---|---|---|
| Characteristics | cowpea trypsin inhibitor (CpTI) (+) | CpTI (−) | Bt/CpTI (+) | Bt/CpTI (−) | Wa |
| Low insect | |||||
| Plant height (cm) | 109.5 ± 1.1a | 105.2 ± 4.4ab | 106.0 ± 2.7a | 111.8 ± 2.4a | 97.0 ± 2.3b |
| No. of tillers | 16.5 ± 0.5a | 17.6 ± 1.1a | 17.2 ± 0.7a | 20.9 ± 1.9ab | 24.0 ± 2.1b |
| No. of panicles | 14.0 ± 0.3a | 14.9 ± 0.7a | 15.7 ± 0.6a | 18.6 ± 1.3b | 20.7 ± 1.3b |
| No. of seeds | 987.9 ± 57.3a | 1032.3 ± 43.4a | 1032.5 ± 76.4a | 1064.5 ± 24.3a | 1065.6 ± 82a |
| Seed set | 60.5 ± 3.0a | 62.5 ± 1.4a | 61.9 ± 2.1a | 58.3 ± 2.3a | 78.3 ± 1.0b |
| 1000-seed weight (g) | 23.4 ± 0.2a | 23.4 ± 0.5a | 22.7 ± 0.2ab | 22.1 ± 0.5b | 20.6 ± 0.3c |
| Natural insect | |||||
| Plant height (cm) | 106.7 ± 1.2a | 106.8 ± 1.5a | 110.3 ± 2.4a | 106.5 ± 2.1a | 98.7 ± 2.6b |
| No. of tillers | 18.8 ± 0.6a | 20.9 ± 1.3ab | 20.3 ± 0.4ab | 18.7 ± 1.1a | 22.2 ± 1.0b |
| No. of panicles | 14.8 ± 0.5a | 14.9 ± 0.9a | 18.9 ± 0.5b | 15.7 ± 0.4a | 18.5 ± 0.8b |
| No. of seeds | 768.0 ± 58.3a | 705.5 ± 38.4a | 1161.6 ± 85.6b | 789.1 ± 27.4a | 807.6 ± 55.7a |
| Seed set | 51.5 ± 1.9a | 50.8 ± 3.1a | 59.3 ± 2.1b | 53.6 ± 1.0ab | 59.1 ± 0.4b |
| 1000-seed weight (g) | 22.3 ± 0.4a | 22.0 ± 0.4a | 22.3 ± 0.2a | 22.0 ± 0.4a | 19.5 ± 0.2b |
Different letters after the means and standard error (±) in the same row indicate significant differences according to Duncan's multiple range tests followed by Bonferroni correction in the same treatment. N = 4 plots.
Performance of F2 hybrid progeny
Both transgenes were associated with reduced insect damage, but this effect was much weaker in the CpTI treatment and was not strong enough to enhance seed production under natural levels of insect pressure (Fig. 2; Appendix 2). However, Bt/CpTI clearly affected fecundity, and the interaction between insect pressure and transgene presence/absence was significant (Fig. 2; Appendix 2). The effects of each transgene on weedy types Wa and Wb were generally similar, so these data are pooled in Fig. 2 and Table 2 for ease of presentation. Bt/CpTI was associated with much lower insect damage and a substantial increase in seed production under both competition treatments (Fig. 2; Appendices 3 and 4). In general, the increase in fecundity in plants with the Bt/CpTI transgene was associated with greater panicle production and percent seed set (Table 2; Appendices 3 and 4).
The insecticide treatment substantially reduced insect damage but did not completely prevent it (Fig. 2; Appendices 3 and 4). Under low insect pressure, ∼5 to 10% of the leaves and tillers on nontransgenic plants sustained damage. For the Bt/CpTI plants, transgene-positive plants had somewhat less insect damage than transgene-negative plants, especially under mixed competition (Fig. 2; Appendices 3 and 4). However, the numbers of seeds per plant were similar in comparisons between transgene-positive versus transgene-negative plants across treatments with low insect pressure.
Performance of F3 hybrid progeny and the weedy parent
Similar effects of the Bt/CpTI transgenes were seen in the F3 hybrid generation (Table 3; Fig. 3; Appendix 5), which was obtained by selfing F2 hybrid progeny from weedy strain Wa. Under natural insect pressure, CpTI had no effect on herbivory or fecundity, while plants in the pure competition treatment with the Bt/CpTI transgene had 79% less insect damage and produced 47% more seeds than their nontransgenic counterparts. An unexpected result in 2009 is the low level of protection from the Bt/CpTI transgene and relatively lower damage on nontransgenic plants in the mixed competition treatment with natural insect pressure. This resulted in similar levels of seed production in comparisons between transgene-positive and transgene-negative plants, in contrast to what we observed in 2008 with F2 plants. The mixed competition treatment is not directly comparable between years because the competitors were different and the plants were closer together in 2009 (see Table 1).
Under low insect pressure, Bt/CpTI was associated with a fitness cost of ∼19% lower fecundity relative to nontransgenic control plants in mixed competition (P < 0.05; Table 1; Fig. 3; Appendix 5); no cost effect was detected in pure cultivation. Plants with the Bt/CpTI transgene had less insect damage than nontransgenic controls, but only under pure competition. Plants with CpTI alone had greater insect damage under mixed competition, counter to expectations. Neither of these differences in insect damage affected fecundity.
We also compared the original weedy parent genotypes directly with their F3 crop–weed hybrid offspring in the pure competition treatment (Table 3). Weedy and hybrid genotypes produced similar numbers of seeds per plant under both natural and low insect treatments, with the exception of the Bt/CpTI F3 plants, which had 79% less insect damage (P < 0.001) and produced 44% (P < 0.01) more seeds than their weedy parents under the natural insect treatment. Thus, fecundity of the F3 hybrid generation was similar to the weedy parent, and we observed a strong benefit of having the Bt/CpTI transgene under natural insect pressure.
Discussion
This study confirms that insect-resistance transgenes from cultivated rice are effective when transferred to weedy rice and can increase fecundity of weedy rice when target insects are present. Results from the F2 and F3 hybrid generations were generally consistent, demonstrating that the Bt/CpTI transgene can result in lower insect damage and greater seed production under natural field conditions, with no effects on survival. The CpTI transgene was also associated with reduced insect damage, but to a much smaller extent than Bt/CpTI, and it was not linked to increased fecundity or survival. Therefore, the benefits of CpTI alone were small relative to those of the Bt transgene. We also found that Bt/CpTI-positive F3 hybrid progeny produced 44% more seeds than their weedy parents in the pure competition treatment. Because fecundity is a key component of fitness, our results suggest that Bt/CpTI transgenes could increase in frequency in weedy rice populations when target insects are sufficiently common, perhaps contributing to larger seed banks and more pervasive weedy rice problems, at least in the short term.
Populations of target insects fluctuate from year to year (e.g. Xia et al. 2010; this study), so the fitness benefit of a Bt/CpTI transgene is expected to vary accordingly. In cultivated rice, lepidopteran herbivores occur in about half of the area of rice cultivation in China (e.g., Sheng et al. 2003), but the extent of lepidopteran damage on weedy rice has not been quantified to our knowledge. However, Cohen et al. (2008) reported similar occurrences of lepidopteran damage on weedy and cultivated rice in the Mekong Delta of Vietnam, suggesting that pests of the crop also attack weedy rice.
As noted previously, the major lepidopteran pests of rice are rice stem borers (S. incertulas, C. suppressalis, and S. inferens) and rice leaf-folder (C. medinalis). These insects also have other host plants; for example, C. suppressalis can feed on Zizania latifolia, maize, sugarcane, and sorghum (Harris 1990; Hou et al. 2009). If non-Bt host crops occur near cultivated rice, this may help delay the evolution of resistant insects by sustaining susceptible genotypes in the area (Carriere et al. 2010). In regions where Bt or Bt/CpTI rice cultivars are planted very widely, and where resistance does not evolve in the target insects, these insect populations may decline dramatically, as documented in Bt cotton (Wu et al. 2008). This could reduce or even eliminate the fecundity advantage of transgene-positive plants. Another ecological factor that could diminish the benefits of Bt/CpTI transgenes is the emergence of secondary insect pests such as hemipterans if they are released from competition with lepidopteran pests. Although complex ecological and evolutionary interactions among rice taxa and their insect herbivores make it challenging to predict long-term fitness consequences of Bt cultivars, our results point to a strong fitness benefit for crop–weed hybrids with Bt/CpTI in the short term when target insect pests are abundant.
A fitness cost was detected in F3 progeny containing Bt/CpTI under conditions where competition between transgene-positive and transgene-negative was introduced. In previous studies with cultivated rice, we observed reduced fecundity of Bt/CpTI in mixed competition plots where GE and non-GE plants competed with each other, but not in monotypic plots, in two of three years with very low insect pressure (Table 1; Appendix 1). Likewise, in the present study, significantly lower fecundity of transgene-positive Bt/CpTI, F3 plants was observed in mixed competition plots with low insect pressure (P < 0.01), and no net gain in fecundity was observed in this treatment combination under natural insect pressure (Table 3; Fig. 3; Appendix 5). This is because of relatively low insect pressure in the mixed-cultivation plots caused by the presence of transgene-positive plants. Therefore, it seems possible that benefits of Bt/CpTI transgenes could be countered by small or moderate fitness costs in years when the target insect populations are very low, but further research involving larger sample sizes is needed to test this hypothesis. The apparent absence of fitness costs associated with transgenes introgressing into wild/weedy populations has also been reported in Brassica rapa and Helianthus annuus (Snow et al. 1999; Burke and Rieseberg 2003; Snow et al. 2003), while an inducible trypsin proteinase inhibitor was associated with a strong fitness cost in Nicotiana attenuata (Zavala et al. 2004).
To our knowledge, this is one of the first studies to document fecundity benefits of transgenes for insect resistance in weedy relatives of a transgenic crop. Similar benefits were seen in wild sunflowers in the USA (Snow et al. 2005), but Bt sunflowers have not been proposed for deregulation (Dalton 2002). In contrast, deployment of Bt rice is already taking place in Iran and China. Although we cannot be sure that our results pertain to other Bt constructs, other weedy rice populations, and other regions, our findings are consistent with the hypothesis that seed production of weedy rice is limited by lepidopteran insect pests, and the introgression of transgenes that confer resistance to these pests can enhance the fitness of recipient populations. Because weedy rice is largely self-pollinating and has high levels of seedling recruitment (Langevin et al. 1990), a small number of crop–wild hybrids with beneficial transgenes could quickly generate transgenic progeny that could then disperse broadly as seeds, while also becoming established in long-lived seed banks. However, we also expect that the widespread cultivation of Bt rice could lead to dramatic declines in local populations of target insects, which may or may not evolve resistance to the Bt toxins in rice. Further studies of annual and regional variation in target insect abundances are needed to gain a deeper understanding of the anticipated prevalence of fitness benefits for Bt or Bt/CpTI weedy rice. To minimize unwanted side effects of growing Bt rice, agricultural practices that reduce weedy rice populations and delay the introgression of crop genes into weedy rice populations, such as hand-weeding prior to seed shattering, could be encouraged in regions where Bt rice is adopted.
Acknowledgments
This work was funded by the ‘973’ program of the Ministry of Science and Technology (2007CB109202, 2011CB100401), the Natural Science Foundation of China (30730066, 30871503), and the National Program of Development of Transgenic New Species of China (2008ZX08011-006). W. Wang, H. B. Xia, and K. Xu of Fudan University kindly provided technical assistance. Data Archiving Statement: Raw data are available in Dryad: http://dx.doi.org/10.5061/dryad.8974.
Appendix 1
Effects of the three transgenic events (Bt, CpTI and Bt/CpTI) on number of seeds per plant in cultivated rice under low versus natural insect pressure and pure versus mixed competition treatments in different years (from Chen et al. 2006 and Xia et al. 2010).
| Insect pressure | cultivation | Treatment | 2003 | 2004 | 2006 |
|---|---|---|---|---|---|
| Low insect | pure | Bt | 259 ± 23.3 | 265.1 ± 39.6 | 724.3 ± 116.5 |
| CpTI | 218 ± 20.1 | 392.6 ± 27.9 | 717.9 ± 29.3 | ||
| Bt/CpTI | 268 ± 14.6+ | 386.0 ± 48.4 | 733.0 ± 84.7 | ||
| Control | 226 ± 17.6 | 312.5 ± 41.9 | 701.4 ± 71.9 | ||
| mixed | Bt-mixed | 273 ± 20.7 | 184.6 ± 32.8 | 663.4 ± 125.7 | |
| Control-1 | 302 ± 33.4 | 386.7 ± 71.6 | 688.5 ± 129.3 | ||
| CpTI-mixed | 246 ± 31.8 | 269.9 ± 91.5 | 609.6 ± 79.4 | ||
| Control-2 | 289 ± 32.2 | 373.9 ± 52.7 | 862.9 ± 137.3 | ||
| Bt/CpTI-mixed | 243 ± 29.0* | 157.8 ± 41.9 | 543.2 ± 136.9* | ||
| Control-3 | 347 ± 30.0 | 339.2 ± 41.6 | 936.3 ± 84.4 | ||
| Natural insect | pure | Bt | 323 ± 28.7* | 701.5 ± 94.0 | 935.5 ± 71.3 |
| CpTI | 288 ± 26.8 | 689.1 ± 48.1 | 1110.3 ± 127 | ||
| Bt/CpTI | 382 ± 31.2** | 729.3 ± 64.8 | 1143.5 ± 68.8* | ||
| Control | 238 ± 24.1 | 732.5 ± 49.5 | 788.6 ± 89.1 | ||
| mixed | Bt-mixed | 360 ± 40.2** | 723.3 ± 70.1 | 691.9 ± 89.9 | |
| Control-1 | 218 ± 26.9 | 736.9 ± 46.4 | 620.4 ± 70.3 | ||
| CpTI-mixed | 270 ± 30.4 | 595.8 ± 101.0 | 710.1 ± 67.8 | ||
| Control-2 | 259 ± 38.8 | 617.3 ± 95.3 | 770.7 ± 98.8 | ||
| Bt/CpTI-mixed | 314 ± 36.1 | 645.2 ± 45.6 | 889.2 ± 78.8 | ||
| Control-3 | 345 ± 48.3 | 628.6 ± 90.6 | 668.8 ± 31.4 |
Plants were grown under low versus natural insect pressure and pure versus mixed competition treatments. Means and SE are shown; Levels of significance: +P < 0.10, *P < 0.05, **P < 0.01.
Appendix 2
Three-way ANOVA for insect damage index and number of seeds per plant in the F2 generation.
| CpTI | Bt/CpTI | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Insect damage | No. of seeds per plant | Insect damage | No. of seeds per plant | ||||||
| Factors | DfN; DfD | F | Sig. | F | Sig. | F | Sig. | F | Sig. |
| Insect pressure | 1; 62 | 107.1 | 0.000 | 38.6 | 0.000 | 141.7 | 0.000 | 22.4 | 0.000 |
| Transgenic genotype | 1; 62 | 4.7 | 0.034 | 0.0 | 0.910 | 117.6 | 0.000 | 8.8 | 0.004 |
| Competition | 1; 62 | 1.3 | 0.258 | 89.4 | 0.000 | 2.5 | 0.122 | 71.6 | 0.000 |
| Insect pressure × Transgenic genotype | 1; 60 | 2.2 | 0.142 | 0.2 | 0.654 | 55.2 | 0.000 | 7.7 | 0.007 |
| Insect pressure × Competition | 1; 60 | 3.3 | 0.075 | 1.8 | 0.187 | 0.7 | 0.420 | 0.1 | 0.718 |
| Transgenic genotype × Competition | 1; 60 | 0.1 | 0.703 | 0.3 | 0.598 | 0.4 | 0.516 | 0.9 | 0.337 |
| Insect pressure × Transgenic genotype × Competition | 1; 56 | 0.2 | 0.637 | 0.3 | 0.583 | 1.3 | 0.260 | 1.7 | 0.197 |
The factors include insect pressure (low versus natural), transgenic genotype (positive versus negative), and competition (pure versus mixed) on insect damage and number of seeds per plant of F2 hybrid progeny for two transgenic events (CpTI or Bt/CpTI) separately. The DFN (numerator degrees of freedom), DFD (denominator degrees of freedom), F value and P value were shown.
Appendix 3
Effects of the two transgenic events (CpTI and Bt/CpTI) on insect damage index and fitness-related traits in the F2 generation (data from weedy strain Wa).
| Wa-CpTI F2 | Wa-Bt/CpTI F2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) |
| Low insect | ||||||||
| Insect index | 12.1 ± 1.5 | 11.1 ± 1.0 | 12.2 ± 1.9 | 11.9 ± 1.2 | 2.4 ± 0.6 | 4.9 ± 1.2 | 5.4 ± 0.6 | 6.8 ± 1.1 |
| Plant height (cm) | 110.4 ± 0.6 | 103.6 ± 5.2 | 106.7 ± 3.7 | 104.5 ± 4.2 | 109.0 ± 3.0 | 115.7 ± 3.2 | 102.8 ± 2.3 | 104.6 ± 2.3 |
| No. of tillers | 19.3 ± 0.8 | 18.6 ± 0.8 | 13.8 ± 1.8 | 14.8 ± 1.4 | 19.3 ± 1.2 | 20.0 ± 1.9 | 14.2 ± 1.8 | 13.8 ± 1.1 |
| No. of panicles | 16.5 ± 0.4 | 16.3 ± 0.6 | 11.6 ± 0.8 | 11.2 ± 0.8 | 17.4 ± 0.7 | 17.0 ± 0.7 | 12.6 ± 2.3 | 10.2 ± 0.4 |
| No. of seeds | 642.5 ± 22.1 | 634.6 ± 31.6 | 464.5 ± 41.2 | 476.7 ± 26.3 | 667.2 ± 43.7 | 719.1 ± 31.3 | 526.4 ± 84.5 | 474.6 ± 18.5 |
| Seed set | 47.2 ± 1.9 | 47.0 ± 0.9 | 46.0 ± 5.0 | 43.9 ± 1.5 | 48.7 ± 2.2 | 53.2 ± 1.5 | 49.3 ± 2.6 | 50.2 ± 2.3 |
| 1000-seed weight (g) | 23.3 ± 0.5+ | 22.2 ± 0.3 | 23.5 ± 1.1 | 22.3 ± 0.9 | 22.0 ± 0.2 | 22.5 ± 0.2 | 21.9 ± 0.5 | 22.7 ± 0.3 |
| Natural insect | ||||||||
| Insect index | 31.8 ± 1.1* | 42.3 ± 2.6 | 29.9 ± 4.8 | 27.7 ± 2.4 | 5.0 ± 0.7** | 22.4 ± 2.4 | 8.8 ± 0.6** | 22.4 ± 2.5 |
| Plant height (cm) | 102.6 ± 1.7* | 94.4 ± 3.0 | 99.7 ± 1.6 | 106.7 ± 5.8 | 117.1 ± 2.4* | 107.8 ± 1.7 | 100.6 ± 2.1 | 99.7 ± 1.9 |
| No. of tillers | 17.8 ± 0.9 | 16.6 ± 0.8 | 13.7 ± 1.0 | 14.7 ± 1.2 | 20.9 ± 0.5* | 18.3 ± 0.6 | 15.0 ± 1.8 | 13.1 ± 1.6 |
| No. of panicles | 14.9 ± 0.8 | 15.1 ± 0.5 | 10.7 ± 1.2 | 11.2 ± 2.2 | 19.3 ± 1.0* | 16.3 ± 0.4 | 14.8 ± 1.4 | 13.0 ± 2.0 |
| No. of seeds | 497.9 ± 29.9 | 490.3 ± 39.4 | 346.1 ± 43.6 | 357.0 ± 43.2 | 726.7 ± 37.0* | 576.1 ± 39.8 | 513.9 ± 44.7* | 390.1 ± 10.3 |
| Seed set | 36.7 ± 1.8 | 36.4 ± 2.1 | 40.3 ± 0.9 | 43.5 ± 4.0 | 47.6 ± 1.3* | 37.8 ± 3.6 | 47.7 ± 2.5* | 39.8 ± 1.3 |
| 1000-seed weight (g) | 21.1 ± 0.3+ | 20.3 ± 0.2 | 21.7 ± 0.8 | 21.3 ± 0.6 | 21.9 ± 0.5* | 20.3 ± 0.4 | 22.4 ± 0.5* | 20.9 ± 0.2 |
Plants were grown under low versus natural insect pressure and pure versus mixed competition treatments. Means and SE are shown; N = 4 plots. Levels of significance: +P < 0.10, *P < 0.05, **P < 0.01.
Appendix 4
Effects of the two transgenic events (CpTI and Bt/CpTI) on insect damage index and fitness-related traits in the F2 generation (data from weedy strain Wb).
| Wb-CpTI F2 | Wb-Bt/CpTI F2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) |
| Low insect | ||||||||
| Insect index | 8.3 ± 0.5 | 11.2 ± 1.3 | 10.0 ± 1.8 | 12.8 ± 1.1 | 4.7 ± 0.6 | 6.5 ± 1.3 | 4.5 ± 0.5* | 8.9 ± 1.1 |
| Plant height (cm) | 101.4 ± 3.3 | 106.0 ± 2.7 | 101.3 ± 3.2 | 106.2 ± 2.4 | 109.4 ± 3.6 | 102.4 ± 4.3 | 110.1 ± 3.9 | 106.5 ± 1.7 |
| No. of tillers | 24.7 ± 1.0 | 24.6 ± 1.4 | 18.4 ± 0.7 | 19.7 ± 0.7 | 28.6 ± 3.4 | 27.3 ± 2.8 | 20.2 ± 1.2 | 19.2 ± 0.6 |
| No. of panicles | 20.5 ± 0.8 | 20.5 ± 1.2 | 12.3 ± 0.6 | 13.7 ± 0.7 | 20.0 ± 1.3 | 20.5 ± 1.1 | 14.7 ± 0.6 | 14.3 ± 1.2 |
| No. of seeds | 621.6 ± 10.9 | 651.6 ± 38.1 | 395.4 ± 27.3 | 403.6 ± 32.4 | 702.6 ± 27.5 | 743.5 ± 54.6 | 583.1 ± 44.8 | 524.6 ± 66.9 |
| Seed set | 45.2 ± 1.8 | 49.8 ± 2.4 | 43.1 ± 3.0 | 44.9 ± 4.2 | 45.7 ± 2.0 | 48.0 ± 1.6 | 51.9 ± 1.7 | 47.1 ± 3.9 |
| 1000-seed weight (g) | 23.3 ± 0.6 | 23.1 ± 0.1 | 21.9 ± 0.6 | 22.8 ± 0.7 | 22.7 ± 0.4 | 23.1 ± 0.5 | 23.0 ± 0.5 | 22.5 ± 0.2 |
| Natural insect | ||||||||
| Insect index | 20.6 ± 0.5 | 24.4 ± 3.6 | 15.9 ± 0.9* | 27.0 ± 2.9 | 8.2 ± 0.8** | 20.9 ± 2.3 | 8.2 ± 1.0* | 19.4 ± 3.1 |
| Plant height (cm) | 95.2 ± 2.5 | 98.2 ± 5.4 | 100.6 ± 1.8 | 97.5 ± 1.8 | 107.3 ± 1.9 | 107.7 ± 2.3 | 113.6 ± 1.6 | 104.4 ± 3.0 |
| No. of tillers | 21.9 ± 1.9 | 22.1 ± 2.0 | 17.5 ± 1.0 | 16.4 ± 1.0 | 24.7 ± 4.5 | 22.2 ± 3.4 | 18.1 ± 1.6 | 17.6 ± 1.1 |
| No. of panicles | 15.3 ± 0.4 | 15.7 ± 0.3 | 11.1 ± 1.1 | 11.4 ± 1.3 | 19.6 ± 1.2* | 16.1 ± 0.4 | 13.9 ± 0.3 | 12.2 ± 1.3 |
| No. of seeds | 520.5 ± 83.0 | 474.8 ± 14.5 | 326.8 ± 24.5 | 343.9 ± 55.8 | 630.4 ± 51.4+ | 509.1 ± 25.5 | 433.7 ± 44.0+ | 315.8 ± 29.5 |
| Seed set | 46.1 ± 3.5 | 43.3 ± 4.2 | 44.0 ± 1.6 | 45.1 ± 2.3 | 49.6 ± 3.4 | 42.0 ± 1.5 | 43.1 ± 1.9* | 35.4 ± 1.6 |
| 1000-seed weight (g) | 22.5 ± 0.3* | 21.5 ± 0.3 | 23.0 ± 0.5 | 22.9 ± 0.6 | 22.5 ± 0.4 | 22.4 ± 0.2 | 22.4 ± 0.5 | 21.8 ± 0.8 |
Plants were grown under low versus natural insect pressure and pure versus mixed competition treatments. Means and SE are shown; N = 4 plots. Levels of significance: +P < 0.10, *P < 0.05, **P < 0.01.
Appendix 5
Effects of the two transgenic events (CpTI and Bt/CpTI) on insect damage index and fitness-related traits in the F3 generation and comparable data from the weedy parent (Wa).
| Wa-CpTI F3 | Wa-Bt/CpTI F3 | Wa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) | Pure (+) | Pure (−) | Mixed (+) | Mixed (−) | Pure |
| Low insect | |||||||||
| Insect index | 10.3 ± 0.9 | 9.6 ± 1.4 | 16.1 ± 1.6+ | 10.7 ± 0.6 | 2.0 ± 0.5** | 5.7 ± 0.4 | 4.0 ± 1.0 | 5.1 ± 0.6 | 6.5 ± 1.2 |
| Plant height (cm) | 109.5 ± 1.1 | 105.2 ± 4.4 | 104.1 ± 3.7 | 98.9 ± 3.0 | 106.0 ± 2.7 | 111.8 ± 2.4 | 105.4 ± 4.0 | 108.1 ± 0.9 | 97.0 ± 2.3 |
| No. of tillers | 16.5 ± 0.5 | 17.6 ± 1.1 | 11.7 ± 0.5 | 11.6 ± 0.6 | 17.2 ± 0.7 | 20.9 ± 1.9 | 12.4 ± 1.2 | 14.9 ± 1.1 | 24.0 ± 2.1 |
| No. of panicles | 14.0 ± 0.3 | 14.9 ± 0.7 | 10.2 ± 0.9 | 9.7 ± 0.6 | 15.7 ± 0.6 | 18.6 ± 1.3 | 10.5 ± 0.6* | 12.6 ± 0.7 | 20.7 ± 1.3 |
| No. of seeds | 987.9 ± 57.3 | 1032.3 ± 43.4 | 609.7 ± 31.2 | 537.9 ± 74.3 | 1032.5 ± 76.5 | 1064.5 ± 24.3 | 580.3 ± 28.4* | 717.9 ± 18.6 | 1065.6 ± 82.0 |
| Seed set | 60.4 ± 2.9 | 62.7 ± 1.4 | 54.8 ± 3.1 | 58.0 ± 3.6 | 61.9 ± 2.1 | 58.3 ± 2.3 | 52.5 ± 2.0 | 55.7 ± 2.4 | 78.3 ± 1.0 |
| 1000-seed weight (g) | 23.4 ± 0.2 | 23.4 ± 0.5 | 23.5 ± 0.2 | 22.7 ± 0.6 | 22.7 ± 0.2 | 22.1 ± 0.5 | 22.9 ± 0.4 | 23.6 ± 0.3 | 20.6 ± 0.3 |
| Natural insect | |||||||||
| Insect index | 21.6 ± 0.8 | 24.9 ± 2.5 | 31.1 ± 2.3 | 27.2 ± 1.6 | 4.9 ± 0.5*** | 22.8 ± 2.2 | 10.5 ± 1.2* | 15.5 ± 2.1 | 22.8 ± 2.7 |
| Plant height (cm) | 106.7 ± 1.2 | 106.8 ± 1.5 | 103.0 ± 1.4* | 100.3 ± 0.9 | 110.3 ± 2.4 | 106.5 ± 2.1 | 111.3 ± 0.9 | 111.4 ± 1.8 | 98.7 ± 2.6 |
| No. of tillers | 18.8 ± 0.6 | 20.9 ± 1.3 | 12.3 ± 0.4 | 11.6 ± 0.7 | 20.3 ± 0.4 | 18.7 ± 1.1 | 13.5 ± 1.3 | 13.4 ± 0.6 | 22.2 ± 1.0 |
| No. of panicles | 14.8 ± 0.5 | 14.9 ± 0.9 | 10.2 ± 0.4 | 10.6 ± 0.3 | 18.9 ± 0.5** | 15.7 ± 0.4 | 11.3 ± 0.6 | 11.6 ± 0.7 | 18.5 ± 0.8 |
| No. of seeds | 768.0 ± 58.3 | 705.5 ± 38.4 | 523.3 ± 68.4 | 474.4 ± 18.7 | 1161.6 ± 85.6** | 789.1 ± 27.4 | 588.4 ± 55.2 | 573.5 ± 54.4 | 807.6 ± 55.7 |
| Seed set | 51.6 ± 1,9 | 51.0 ± 3.1 | 53.9 ± 4.3 | 52.4 ± 1.2 | 59.3 ± 2.1+ | 53.6 ± 1.0 | 58.2 ± 1.5 | 55.1 ± 2.7 | 59.1 ± 0.4 |
| 1000-seed weight (g) | 22.3 ± 0.4 | 22.0 ± 0.4 | 22.1 ± 0.4 | 21.9 ± 0.6 | 22.3 ± 0.2 | 22.0 ± 0.4 | 22.4 ± 0.2 | 21.4 ± 0.6 | 19.5 ± 0.2 |
Plants were grown under low versus natural insect pressure and pure versus mixed competition treatments. Means and SE are shown; N = 4 plots.
Levels of significance: +P < 0.10, *P < 0.05, **P < 0.01, ***P < 0.001.
Author contributions
Conceived and designed the experiments: XY, BL, HX. Performed the experiments: XY, HX, WW, FW, JS. Analyzed the data: XY, BL, AS. Wrote the paper: XY, BL, AS, HX.
Literature cited
- Ammitzbøll H, Mikkelsen TN, Jørgensen RB. Transgene expression and fitness of hybrids between GM oilseed rape and Brassica rapa. Environmental Biosafety Research. 2005;4:3–12. doi: 10.1051/ebr:2005010. [DOI] [PubMed] [Google Scholar]
- Andow DA, Zwahlen C. Assessing environmental risks of transgenic plants. Ecology Letters. 2006;9:196–214. doi: 10.1111/j.1461-0248.2005.00846.x. [DOI] [PubMed] [Google Scholar]
- Arrieta-Espinoza G, Sanchez E, Vargas S, Lobo J, Quesada T, Espinoza AM. The weedy rice complex in Costa Rica. I. Morphological study of relationships between commercial rice varieties, wild Oryza relatives and weedy types. Genetic Resources and Crop Evolution. 2005;52:575–587. [Google Scholar]
- Burke JM, Rieseberg LH. Fitness effects of transgenic disease resistance in sunflowers. Science. 2003;300:1250. doi: 10.1126/science.1084960. [DOI] [PubMed] [Google Scholar]
- Cao QJ, Xia H, Yang X, Lu BR. Performance of hybrids between weedy rice and insect-resistant transgenic rice under field experiments: implication for environmental biosafety assessment. Journal of Integrative Plant Biology. 2009;51:1138–1148. doi: 10.1111/j.1744-7909.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- Carriere Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evolutionary Applications. 2010;3:561–573. doi: 10.1111/j.1752-4571.2010.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Lee DS, Song ZP, Suh HS, Lu BR. Gene flow from cultivated rice (Oryza sativa) to its weedy and wild relatives. Annals of Botany. 2004;93:67–73. doi: 10.1093/aob/mch006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Snow AA, Wang F, Lu BR. Effects of insect-resistance transgenes on fecundity in rice (Oryza sativa, Poaceae): a test for underlying costs. American Journal of Botany. 2006;93:94–101. [Google Scholar]
- Cohen MB, Arpaia S, Lan LP, Chau LM, Snow AA. Shared flowering phenology, insect pests, and pathogens among wild, weedy, and cultivated rice in the Mekong Delta, Vietnam: implications for transgenic rice. Environmental Biosafety Research. 2008;7:73–85. doi: 10.1051/ebr:2008011. [DOI] [PubMed] [Google Scholar]
- Dalton R. Superweed study falters as firms deny access to transgene. Nature. 2002;419:655. doi: 10.1038/419655a. [DOI] [PubMed] [Google Scholar]
- Delouche JC, Burgos NR, Gealy DR, Zorilla-San MG, Labrada R, Larinde M. Rome: FAO of the United Nations; 2007. Weedy rices – origin, biology, ecology and control; p. 144. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin. 1987;19:11–15. [Google Scholar]
- Ellstrand NC. Current knowledge of gene flow in plants: implications for transgene flow. Philosophical Transactions of the Royal Society B-Biological Sciences. 2003;358:1163–1170. doi: 10.1098/rstb.2003.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2004. The state of food and agriculture 2003–2004. Agricultural biotechnology: meeting the needs of the poor?
- Gealy DR, Mitten DH, Rutger JN. Gene flow between red rice (Oryza sativa) and herbicide-resistant rice (O. sativa): Implications for weed management. Weed Technology. 2003;17:627–645. [Google Scholar]
- Gealy DR, Yan WG, Rutger JN. Red rice (Oryza sativa) plant types affect growth, coloration, and flowering characteristics of first- and second-generation crosses with rice. Weed Technology. 2006;20:839–852. [Google Scholar]
- Godfree RC, Thrall PH, Young AG. Enemy release after introduction of disease-resistant genotypes into plant - pathogen systems. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2756–2760. doi: 10.1073/pnas.0608356104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressel J. Transgenics are imperative for biofuel crops. Plant Science. 2008;174:246–263. [Google Scholar]
- Gressel J, Valverde BE. A strategy to provide long-term control of weedy rice while mitigating herbicide resistance transgene flow, and its potential use for other crops with related weeds. Pest Management Science. 2009;65:723–731. doi: 10.1002/ps.1754. [DOI] [PubMed] [Google Scholar]
- Hall L, Topinka K, Huffman J, Davis L, Good A. Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Science. 2000;48:688–694. [Google Scholar]
- Harris KM. Bioecology of Chilo species. Insect Science and Its Application. 1990;11:467–477. [Google Scholar]
- Harlan JR, Wet JMJd. Toward a rational classification of cultivated plants. Taxon. 1971;20:509–517. [Google Scholar]
- Hou ML, Lin W, Han YQ. Seasonal changes in supercooling points and glycerol content in overwintering larvae of the Asiatic rice borer from rice and water-oat plants. Environmental Entomology. 2009;38:1182–1188. doi: 10.1603/022.038.0427. [DOI] [PubMed] [Google Scholar]
- James C. ISAAA Brief No. 35. NY: ISAAA: Ithaca; 2006. Global Status of Commercialized Biotech/GM Crops: 2006. [Google Scholar]
- James C. Global Status of Commercialized Biotech/GM Crops: 2009. Ithaca, NY, USA: ISAAA Brief No. 41 ISAAA; 2009. [Google Scholar]
- Jenczewski E, Ronfort J, Chèvre A-M. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environmental Biosafety Research. 2003;2:9–24. doi: 10.1051/ebr:2003001. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bellinder RR, Brainard DC, Malik RK, Gupta RK. Risks of herbicide-resistant rice in India: a review. Crop Protection. 2008;27:320–329. [Google Scholar]
- Langevin SA, Clay K, Grace JB. The incidence and effects of hybridization between cultivated rice and its related weed red rice (Oryza sativa L.) Evolution. 1990;44:1000–1008. doi: 10.1111/j.1558-5646.1990.tb03820.x. [DOI] [PubMed] [Google Scholar]
- Laughlin KD, Power AG, Snow AA, Spencer LJ. Risk assessment of genetically engineered crops: fitness effects of virus-resistance transgenes in wild Cucurbita pepo. Ecological Applications. 2009;19:1091–1101. doi: 10.1890/08-0105.1. [DOI] [PubMed] [Google Scholar]
- Londo JP, Schaal BA. Origins and population genetics of weedy red rice in the USA. Molecular Ecology. 2007;16:4523–4535. doi: 10.1111/j.1365-294X.2007.03489.x. [DOI] [PubMed] [Google Scholar]
- Lu BR, Snow AA. Gene flow from genetically modified rice and its environmental consequences. BioScience. 2005;55:669–678. [Google Scholar]
- Messeguer J, Marfa V, Catala MM, Guiderdoni E, Mele E. A field study of pollen-mediated gene flow from Mediterranean GM rice to conventional rice and the red rice weed. Molecular Breeding. 2004;13:103–112. [Google Scholar]
- National Research Council. Washington, DC: US National Academies Press; 2002. Environmental effects of transgenic plants: the scope and adequacy of regulation. (also see http://www.ventria.com/news/Press%20Release%209-29-06.asp accessed on 2 October 2010) [PubMed] [Google Scholar]
- Oard J, Cohn MA, Linscombe S, Gealy D, Gravois K. Field evaluation of seed production, shattering, and dormancy in hybrid populations of transgenic rice (Oryza sativa) and the weed, red rice (Oryza sativa. Plant Science. 2000;157:13–22. doi: 10.1016/s0168-9452(00)00245-4. [DOI] [PubMed] [Google Scholar]
- Olofsdotter M, Valverde BE, Madsen KH. Herbicide resistant rice (Oryza sativa L.): global implications for weedy rice and weed management. Annals of Applied Biology. 2000;137:279–295. [Google Scholar]
- Rong J, Song ZP, Su J, Xia H, Lu BR, Wang F. Low frequency of transgene flow from Bt/CpTI rice to its nontransgenic counterparts planted at close spacing. New Phytologist. 2005;168:559–566. doi: 10.1111/j.1469-8137.2005.01539.x. [DOI] [PubMed] [Google Scholar]
- Sasu MA, Ferrari MJ, Du DL, Winsor JA, Stephenson AG. Indirect costs of a nontarget pathogen mitigate the direct benefits of a virus-resistant transgene in wild Cucurbita. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19067–19071. doi: 10.1073/pnas.0905106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng CF, Wang HT, Sheng SY, Gao LY, Xuan WJ. Pest status and loss assessment of crop damage caused by the rice borers, Chilo suppressalis and Tryporyza ineertulas in China. Entomological Knowledge. 2003;40:289–294. [Google Scholar]
- Shivrain VK, Burgos NR, Gealy DR, Sales MA, Smith KL. Gene flow from weedy red rice (Oryza sativa L.) to cultivated rice and fitness of hybrids. Pest Management Science. 2009;65:1124–1129. doi: 10.1002/ps.1802. [DOI] [PubMed] [Google Scholar]
- Snow AA, Andersen B, Jorgensen RB. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy Brassica rapa. Molecular Ecology. 1999;8:605–615. [Google Scholar]
- Snow AA, Pilson D, Rieseberg LH, Paulsen MJ, Pleskac N, Reagon MR, Wolf DE, et al. A Bt transgene reduces herbivory and enhances fecundity in wild sunflowers. Ecological Applications. 2003;13:279–286. [Google Scholar]
- Snow AA, Andow DA, Gepts P, Hallerman EM, Power A, Tiedje JM, Wolfenbarger LL. Genetically engineered organisms and the environment: current status and recommendations. Ecological Applications. 2005;15:377–404. [Google Scholar]
- USDA. 2006. US Deppartment of Agriculture deregulates LL601 rice variety. Available at: http://www.aphis.usda.gov/newsroom/content/2006/11/rice_deregulate.shtml (accessed on 10 September 2010)
- Wu KM, Lu YH, Feng HQ, Jiang YM, Zhao JZ. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science. 2008;321:1676–1678. doi: 10.1126/science.1160550. [DOI] [PubMed] [Google Scholar]
- Xia H, Lu BR, Su J, Chen R, Rong J, Song ZP, Wang F. Normal expression of insect-resistant transgene in progeny of common wild rice crossed with genetically modified rice: its implication in ecological biosafety assessment. Theoretical and Applied Genetics. 2009;119:635–644. doi: 10.1007/s00122-009-1075-5. [DOI] [PubMed] [Google Scholar]
- Xia H, Chen LY, Wang F, Lu BR. Yield benefit and underlying cost of insect-resistance transgenic rice: implication in breeding and deploying transgenic crops. Field Crops Research. 2010;118:215–220. [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT. Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1607–1612. doi: 10.1073/pnas.0305096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang NY, Linscombe S, Oard J. Out-crossing frequency and genetic analysis of hybrids between transgenic glufosinate herbicide-resistant rice and the weed, red rice. Euphytica. 2003;130:35–45. [Google Scholar]
- Zhu B, Lawrence JR, Warwick SI, Mason P, Braun L, Halfhill MD, Stewart CN. Stable Bacillus thuringiensis (Bt) toxin content in interspecific F1 and backcross populations of wild Brassica rapa after Bt gene transfer. Molecular Ecology. 2004;13:237–241. doi: 10.1046/j.1365-294x.2004.02018.x. [DOI] [PubMed] [Google Scholar]


