Abstract
At Odense University Hospital (OUH), 5–9% of all unselected cardiac surgical patients undergo reoperation due to excessive bleeding. The reoperated patients have an approximately three times greater mortality than non-reoperated. To reduce the rate of reoperations and mortality due to postoperative bleeding, we aim to identify risk factors that predict reoperation. A total of 1452 consecutive patients undergoing cardiac surgery using extracorporeal circulation (ECC) between November 2005 and December 2008 at OUH were analysed. Statistical tests were used to identify risk factors for reoperation. We performed a case-note review on propensity-matched patients to assess the outcome of reoperation for bleeding regarding morbidity and mortality. In total, 101 patients (7.0%) underwent surgical re-exploration due to excessive postoperative bleeding. Significant risk factors for reoperation for bleeding after cardiac surgery was low ejection fraction, high EuroSCORE, procedures other than isolated CABG, elongated time on ECC, low body mass index, diabetes mellitus and preoperatively elevated s-creatinine. Reoperated patients significantly had a greater increase in postoperative s-creatinine and higher mortality. Surviving reoperated patients significantly had a lower EuroSCORE and a shorter time on ECC compared with non-survivors. The average time to re-exploration was 155 min longer for non-survivors when compared with survivors.
Keywords: Cardiac surgery, Reoperation, Postoperative bleeding, Risk factors
INTRODUCTION
Risk of reoperation due to bleeding after cardiac surgery is shown to be 2.2–4.2% [1–6]. Risk factors are high age, low body mass index (BMI) or body surface area (BSA), time on extracorporeal circulation (ECC), five or more anastomosis or non-elective operation [2–4]. Reoperated patients have a two to six times greater mortality [1, 3–5] and a greater morbidity regarding renal and pulmonary function, sepsis and arrhythmia [1, 4, 5]. Postoperative bleeding can be due to surgical or coagulopathic factors [1]. Patient-related factors are also indicative for reoperation due to bleeding.
At Odense University Hospital (OUH), 5–9% of all unselected cardiac surgical patients undergo reoperation due to excessive bleeding. The reoperated patients had three times greater mortality and, to lower the rate of reoperation due to bleeding and improve the mortality, we have tried to identify risk factors in patients as well as in procedures.
MATERIALS AND METHODS
Patient population and data
Patient data were collected consecutively from The Western Denmark Heart Registry [7] (WDHReg) during 18 November 2005 until 31 December 2008 at OUH. During this period, we included all patients undergoing cardiac surgery using ECC. Of 1452 patients found, 101 patients (7.0%) underwent at least one reoperation within 24 h due to excessive postoperative bleeding. Pre-, peri- and postoperative characteristics were collected from WDHReg complemented by case records. Information included the patient's age, type of operation, complications, bleeding and possible causes of death. We qualified risk factors for reoperation by comparing the results of 101 reoperated patients with the remaining 1351 patients.
Using recorded operative details, we classified the bleeding as coagulopathic or surgical. Blood or haematoma without ongoing bleeding and/or diffuse oozing was defined as coagulopathic bleeding. Specific bleeding requiring clips or suture was defined as surgical bleeding.
Propensity-matched group
To assess the outcome of reoperation for bleeding regarding morbidity and mortality, we performed a case-note review on propensity-matched patients. Patients requiring re-exploration for bleeding were propensity matched with a unique patient not re-explored using the following criteria: priority of the operation, age, BMI, medicine (acetylsalicylic acid, clopidogrel, coumadine or heparin) within the last 5 days, EuroSCORE (European System for Cardiac Operative Risk Evaluation) and sex. When possible we also matched patients with regard to ejection fraction (EF), diabetes mellitus (DM) and the type of procedure. The mentioned criteria are the result of a literature study [2, 5]. Matching the patients using these criteria, we minimized their influence as confounders when analysing the consequences of reoperations.
Mortality was defined as death within 30 days after the first operation. Complications such as perioperative myocardial infarction, transient cerebral ischaemic attack (TIA), stroke and sternal infection had to be diagnosed by a doctor within the same hospital admission as the index surgery. The morbidity definitions were according to the definitions of the protocol at WDHReg [7]. Stroke is defined as a neurological deficit of cerebrovascular cause that persists beyond 24 h or is interrupted by death within 24 h. TIAs share the same aetiology as strokes, but the symptoms resolve within 24 h. Sternal infections often appeared after the discharge, so we searched the case records after discharge.
The decision to perform resternotomy was made by the surgeon on call and based on conventional guidelines: (i) drainage of >500 ml in the first hour, total drainage of >800 ml in the first 2 h, 900 ml in the first 3 h, >1000 ml in the first 4 h or 1200 ml in the first 5 h; (ii) sudden massive bleeding or cardiac tamponade.
Statistical analysis
Differences between the two groups were explored using an independent t-test for continuous variables and Fisher's exact test and Pearson's χ2 test for categorical variables. Data were given as mean values, standard deviations of mean values and in numbers and percentages. In all cases, a P-value <0.05 was considered as significant.
Statistical analysis was performed with SPSS (SPSS Inc., PASW Statistics 18).
RESULTS
Causes of death were divided into circulatory, multi-organ failure, bowel ischaemia and unknown (see Fig. 1).
Figure 1:
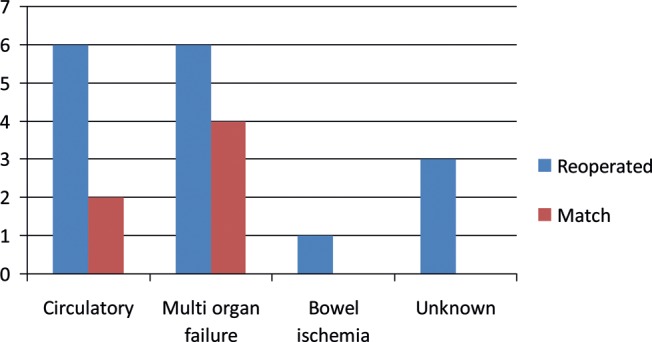
Causes of death among the 16 reoperated and 6 matched patients.
The total population
Of the 1452 patients undergoing on-pump cardiac surgery during the study period, 101 patients (7.0%) underwent a surgical re-exploration for postoperative bleeding (types of bleeding, Fig. 2).
Figure 2:
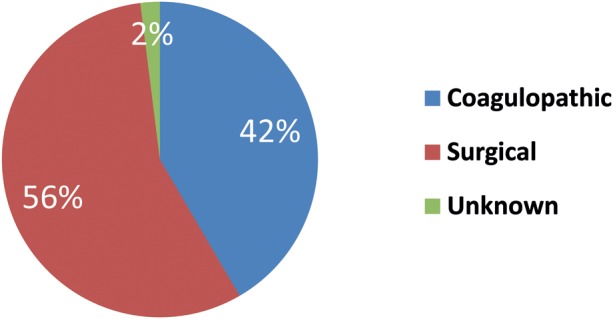
Types of bleeding found at reoperation. Forty-two patients had a coagulopathic bleeding, 57 patients had a surgical bleeding and 2 patients had an unknown type of bleeding as it was not described further in the operation description.
Mortality in patients re-explored for surgical bleeding and coagulopathic bleeding was 19 and 10.5%, respectively.
Patients in the re-explored group had a higher mortality when compared with patients who were not re-explored (15.8 versus 5.7%, P < 0.001). Predictors for reoperation due to bleeding are listed in Table 1.
Table 1:
Risk factors for reoperation
| Variables | Reoperated (n = 101), mean ± SD or number (%) | Not reoperated (n = 1351), mean ± SD or number (%) | P-value |
|---|---|---|---|
| Age (year) | 67.0 ± 11.6 | 66.1 ± 10.6 | 0.414 |
| Sex (male) | 73 (72.3) | 984 (72.8) | 0.908 |
| Priority (acute) | 15 (14.9) | 125a (9.3) | 0.079 |
| EuroSCORE | 12.2 ± 13.1 | 8.3 ± 11.7 | 0.001 |
| EF | 48.5 ± 12.9 | 51.3 ± 11.8 | 0.022 |
| Procedure: isolated CABG | 25 (24.8) | 564 (41.7) | 0.001 |
| Time on ECC (min) | 158.3 ± 83.9 | 126.7 ± 57.6 | 0.000 |
| BMI (kg/m2) | 25.4 ± 4.0 | 27.1 ± 4.4 | 0.000 |
| DM | 25 (25.8) | 199a (14.7) | 0.010 |
| Hypertension | 51 (50.5) | 596a (44.4) | 0.255 |
| Previous AMI | 31 (30.7) | 395a (29.3) | 0.821 |
| Previous PCI | 16 (15.8) | 176a (13.1) | 0.447 |
| Medicine within 5 days | 23 (22.8) | 302 (22.4) | 0.902 |
| Creatinine >134 µmol/l at admission | 17 (16.8) | 105a (7.8) | 0.004 |
aNumber (n) different from 1351 due to missing data. Results are calculated with another ‘n’. Priority, DM, creatinine and epidural, n = 1350, hypertension, n = 1341; previous AMI, n = 1346; previous PCI, n = 1340.
Matched group
The group of reoperated patients and propensity-matched group were homogeneous with respect to preoperative and perioperative variables. However, preoperative s-creatinine was significantly higher in the re-explored group when compared with the control group (Table 2).
Table 2:
Comparison of case and match group
| Variables | Reoperated (n = 101), mean ± SD or number (%) | Match (n = 101), mean ± SD or number (%) | P-value |
|---|---|---|---|
| Priority (acute) | 15 (14.9) | 15 (14.9) | 1.000 |
| Age (year) | 67.0 ± 11.6 | 67.0 ± 11.6 | 0.990 |
| BMI (kg/m2) | 25.4 ± 4.0 | 25.6 ± 4.2 | 0.711 |
| EuroSCORE | 12.2 ± 13.1 | 13.2 ± 16.1 | 0.647 |
| Medicine within 5 days | 23 (22.8) | 23 (22.8) | 1.000 |
| Sex (male) | 73 (72.3) | 72 (71.3) | 1.000 |
| EF | 48.5 ± 12.9 | 49.1 ± 12.4 | 0.710 |
| DM | 25 (24.8) | 17 (16.8) | 0.225 |
| CABG | 59 (58.4) | 58 (57.4) | 1.000 |
| Aortic valve | 55 (54.5) | 42 (41.6) | 0.091 |
| Mitral valve | 11 (10.9) | 17 (16.8) | 0.309 |
| Other procedure | 33 (32.7) | 22 (21.8) | 0.114 |
| S-creatinine (µmol/l) | 109.8 ± 39.4 | 97.6 ± 24.2 | 0.008 |
Patients undergoing re-exploration had higher mediastinal drainage in the intensive care unit (2905.3 versus 829.9 ml). Furthermore, the mortality rate was higher in the re-explored group (15.8 versus 5.9%) (Table 3).
Table 3:
Comparing the outcome between reoperated and match group
| Variables | Reoperated (n = 101), Mean ± SD or number (%) | Match (n = 101), Mean ± SD or number (%) | P-value |
|---|---|---|---|
| TIAa | 2 (2.0) | 0 (0.0) | 0.129 |
| Strokea | 0 (0.0) | 3 (3.0) | |
| Lacunar infarcta | 3 (3.0) | 1 (1.0) | |
| Sternal infection | 0 (0.0) | 1 (1.0) | 1.000 |
| AMI | |||
| Yes | 4 (4.0) | 4 (4.0) | 0.929 |
| Uncertain | 4 (4.9) | 3 (3.0) | |
| Mortality | 16 (15.8) | 6 (5.9) | 0.040 |
| Drainage during the admission at intensive care unit (ml)b | 2905.3 ± 2332.9 | 829.9 ± 626.3 | 0.000 |
aNumber different from 202; reoperated 99, match 100.
bNumber different from 202, 91 in both groups.
To analyse the impact of re-exploration on the renal function, we excluded patients with preoperatively elevated s-creatinine (s-creatinine > 134 µmol/l at admission). This reduced the group of re-explored patients from 101 to 82 patients and the control group was reduced to 97. The increase in postoperative s-creatinine is greater for the re-explored when compared with the control group (P = 0.001). The maximum value of s-creatinine is also different in the two groups (160.4 versus 126.1 µmol/l) (Table 4).
Table 4:
Comparing s-creatinine between reoperated and match
| Variables | Reoperated (n = 82), mean ± SD | Match (n = 97), mean ± SD | P-value |
|---|---|---|---|
| S-creatinine admission (µmol/l) | 96.1 ± 16.8 | 94.3 ± 16.4 | 0.476 |
| S-creatinine max | 160.4 ± 89.4 | 126.1 ± 47.3 | 0.001 |
| S-creatinine discharge/death | 107.5 ± 39.2 | 103.5 ± 32.8 | 0.458 |
| Average increase | 64.3 ± 84.2 | 31.8 ± 44.2 | 0.001 |
| Difference discharge-admission | 11.5 ± 33.1 | 9.2 ± 28.0 | 0.626 |
Patients with creatinine above 134 at admission were excluded from this comparison. Furthermore, two patients are excluded due to missing data.
Surviving patients in the re-explored group had a lower EuroSCORE than non-survivors (P = 0.010). Additionally, a significant difference in the re-explored group is a longer ECC time. On average, the surviving patients were re-explored 155 min earlier than the others (561.4 versus 406.4 min) (Table 5).
Table 5:
Comparison of reoperated survivors and non-survivors
| Variables | Non-survivors (n = 16), mean ± SD or number (%) | Survivors (n = 85), mean ± SD or number (%) | P-value |
|---|---|---|---|
| Sex (male) | 12 (75.0) | 61 (71.8) | 1.000 |
| Age (year) | 70.8 ± 5.8 | 66.3 ± 12.3 | 0.151 |
| EuroSCORE | 19.9 ± 20.0 | 10.8 ± 10.9 | 0.010 |
| BMI (kg/m2) | 26.7 ± 5.1 | 25.1 ± 3.9 | 0.166 |
| EF | 43.8 ± 12.1 | 49.3 ± 13.0 | 0.114 |
| DM | 5 (31.3) | 20 (23.5) | 0.535 |
| Priority (acute) | 3 (18.8) | 12 (14.1) | 0.702 |
| Medicine <5 days | 4 (20.0) | 19 (22.4) | 0.756 |
| ECC-time (min) | 216.7 ± 92.7 | 147.3 ± 77.9 | 0.002 |
| S-creatinine admission (µmol/l) | 127.4 ± 42.5 | 106.5 ± 38.2 | 0.052 |
| S-creatinine maxa | 226.6 ± 84.1 | 172.4 ± 105.4 | 0.081 |
| S-creatinine increasea | 97.0 ± 66.8 | 68.6 ± 92.3 | 0.290 |
| Type of bleeding (surgical) | 0.256 | ||
| Isolated CABG | 3 (18.8) | 22 (25.9) | 1.000 |
| Drainageb | 2049.3 ± 1135.1 | 1783.5 ± 1049.6 | 0.376 |
| Time to reoperation (min)c | 561.4 ± 331.1 | 406.4 ± 275.4 | 0.061 |
a97 patients; 13 non-survivors and 84 survivors.
b95 patients; 15 non-survivors and 80 survivors, drainage until reoperation.
c99 patients; 14 non-survivors and 85 survivors.
One surgeon had a greater risk for re-exploration compared with the other surgeons (surgeon 12, P = 0.003) (Fig. 3), but no difference in mortality was seen among the surgeons.
Figure 3:
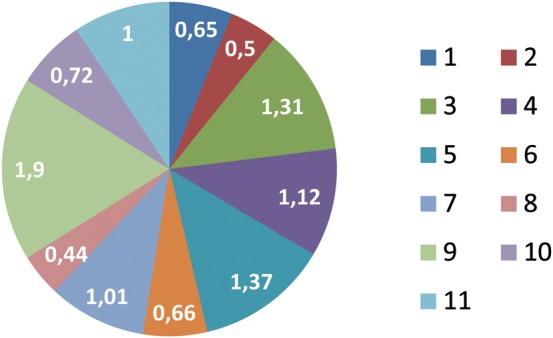
Relative risk for reoperation with respect to the surgeon. The surgeons’ identification numbers are listed to the right. The relative risk is shown in the figure.
DISCUSSION
Of 1452 patients undergoing cardiac surgery, 101 patients (7.0%) required at least one reoperation within 24 h due to excessive bleeding. This rate is greater than previously reported rates of 2.2–4.2% [1–6] and also greater than the target rate of the surgical ward of 5.0%. It has not been possible for the surgeons to stay within the recommended rates over the examined 3-year period, but the rate is no greater than the national average rate for Denmark in the given period estimated by WDHReg [7].
It has been shown that reoperation is associated with an increased mortality [1, 3–6]. Our study confirmed this by showing an increase in mortality by three times (2.7–2.8) for re-explored patients compared with both all non-re-explored and matched control group.
We identified seven significant pre- and intraoperative variables that were predictive for re-exploration due to bleeding: low BMI; low EF; high EuroSCORE; preoperatively increased creatinine; DM; procedures other than coronary artery bypass graft surgery (CABG) and prolonged time on ECC. These variables are intertwined in various ways. Other studies have found age and priority associated with the risk of reoperation, but our study did not show this association [2–4]. We did not find any significant association between a medical history with hypertension or previous AMI/PCI (respectively, acute myocardial infarction and percutaneous coronary intervention) and risk for reoperation. Interestingly we found that patients with DM have a significantly greater risk for reoperation due to bleeding which is not seen in other studies [2–4]. Thus the risk for reoperation seems to depend on the type of preoperative morbidity the patients present themselves with.
Karthik et al. [2] studied 2898 patients undergoing CABG with a reoperation rate of 3.1% to identify risk factors for reoperation. Variables such as low BMI, non-elective surgery, increasing number of grafts and increasing age was identified as having a significantly greater risk for reoperation for bleeding. Moulton et al. [4] studied 6015 patients undergoing cardiac surgery with a reoperation rate of 4.2%. High age, preoperative renal insufficiency, procedures other than CABG and prolonged time on ECC were at greater risk of needing reoperation for bleeding. Dacey et al. [3] studied 8586 patients with a reoperation rate of 3.6%. In this substantial study, high rates of re-exploration for haemorrhage were observed in patients with increasing age, smaller BSA, prolonged time on ECC and number of grafts.
We differentiated the haemorrhage in coagulopathic and surgical to assess whether it had an influence on mortality or not. While 56.4% of the patients had surgical bleeding, the remaining 41.6% were coagulopathic bleeding. Mortality was 19.0% for patients with surgical bleeding, and 10.5% for patients with coagulopathic bleeding; this difference is not significant (P = 0.256). Hall et al. [1] differentiated the haemorrhage between surgical and coagulopathic to determine the differences in patients’ outcome. Of the 2263 patients undergoing cardiac surgery, 3.6% were reoperated due to bleeding. While 66% had a surgical bleeding, the remaining 34% had coagulopathic bleeding. The mortality for reoperated group was 8.7 and 12.5%, respectively, and for non-reexplored it was 2%. Our study did not find the same tendency as Hall et al.'s study regarding mortality as our patients with surgical bleedings had a greater mortality than the coagulopathic.
Morbidity could be examined using the propensity-matched score. We did not find a significant difference in morbidity between the re-explored group compared with the propensity-matched group regarding AMI, sternal infection, stroke and the level of creatinine after discharge. This is similar to the findings from Karthik et al. [2]. It should be stressed that these postoperative complications are infrequent and a large number of patients are needed to show a difference.
Focusing on renal function we excluded patients in both the reoperated group and the propensity-matched group with preoperative elevated creatinine (>134 µmol/l). Having only patients with normal pre-operative creatinine in both groups, we evaluated the level of postoperative rise of s-creatinine, and patients reoperated for bleeding had a greater increase in s-creatinine both average and maximum which was significant. Surprisingly, there was no difference in the values at discharge/death between the two groups.
Among the patients undergoing reoperation the prolonged ECC time was associated with increased mortality. We did not find any significant difference regarding the time to re-exploration between the reoperated survivors and non-survivors, but one could consider that it might make a difference if the patients underwent earlier re-exploration. The average time to re-exploration in the two groups was 561 and 406 min, respectively. This is in conflict with the guidelines regarding postoperative bleeding and reoperation outlining the volume of bleeding up to 5 h (300 min) after the first operation.
CONCLUSIONS
Our aim was to find factors with significant influence on the risk for reoperation due to haemorrhage after cardiac surgery and evaluate the consequence of the reoperation. Like several other studies, we found a higher mortality for reoperated patients. We also found that low EF, high EuroSCORE, procedures other than CABG, the time on ECC, low BMI and preoperatively elevated s-creatinine had a significant influence on the risk for reoperation. These variables are intertwined in various ways. We found a significant association between DM and the risk for reoperation.
By comparing the reoperated patients with a propensity-matched group, we found that the reoperated patients had a significantly greater increase in postoperative s-creatinine.
By analysing the reoperated patients we found that the group of surviving patients had a lower EuroSCORE, a shorter time on the ECC and a shorter time to re-exploration.
The haemorrhage was differentiated in coagulopathic and surgical. In total, 56.4% of the patients had surgical bleeding.
Patients with high EuroSCORE, low EF, low BMI, DM, preoperative s-creatinine >134 µmol/l and procedures other than CABG should have a very carefully planned operation. Preoperatively, discontinuation of pertinent medication and screening coagulation in blood samples could reduce coagulopathic bleeding. Initiatives such as checklists, action cards, guidelines and regular audits can help reduce surgical causes for reoperation due to bleeding. It is mandatory to strictly follow guidelines regarding reoperation for postoperative bleeding and thereby possibly reduce the amount of time and blood spent before performing a necessary reoperation.
Conflict of interest: none declared.
REFERENCES
- 1.Hall TS, Brevetti GR, Skoultchi AJ, Sines JC, Gregory P, Spotnitz AJ. Reexploration for hemorrhage following open heart surgery differentiation on the causes of bleeding and the impact on patient outcomes. Ann Thorac Cardiovasc Surg. 2001;7:352–7. [PubMed] [Google Scholar]
- 2.Karthik S, Grayson AD, McCarron EE, Pullan DM, Desmond MJ. Reexploration for bleeding after coronary artery bypass surgery: risk factors, outcomes, and the effect of time delay. Ann Thorac Surg. 2004;78:527–34. doi: 10.1016/j.athoracsur.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 3.Dacey LJ, Munoz JJ, Baribeau YR, Johnson ER, Lahey SJ, Leavitt BJ, et al. Reexploration for hemorrhage following coronary artery bypass grafting: incidence and risk factors. Northern New England Cardiovascular Disease Study Group. Arch Surg. 1998;133:442–7. doi: 10.1001/archsurg.133.4.442. [DOI] [PubMed] [Google Scholar]
- 4.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg. 1996;111:1037–46. doi: 10.1016/s0022-5223(96)70380-x. [DOI] [PubMed] [Google Scholar]
- 5.Ranucci M, Bozzetti G, Ditta A, Cotza M, Carboni G, Ballotta A. Surgical reexploration after cardiac operations: why a worse outcome? Ann Thorac Surg. 2008;86:1557–62. doi: 10.1016/j.athoracsur.2008.07.114. [DOI] [PubMed] [Google Scholar]
- 6.Gwozdziewicz M, Olsak P, Lonsky V. Re-operations for bleeding in cardiac surgery: treatment strategy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:159–62. doi: 10.5507/bp.2008.026. [DOI] [PubMed] [Google Scholar]
- 7. The Danish Heart Register. www.dhreg.dk. (8 February 2010, date last accessed)


