Abstract
We report the case of a patient with a pulmonary artery (PA) aneurysm secondary to a regurgitant quadricuspid pulmonary valve, in which both lesions were successfully repaired. The patient, a 16-year old boy, was known to have had pulmonary regurgitation and progressive dilation of the PA for years. He was operated on when he developed symptoms of effort, a dilated right ventricle and a PA of 55 mm. The quadricuspid pulmonary valve was an intraoperative finding. It had a dilated annulus, two normal cusps, a third with a ‘sparrow-nest’ configuration and the fourth was severely hypoplastic (which explained the valve regurgitation). The valve was repaired through tricuspidisation of the quadricuspid pulmonary valve (annular plication at the level of the hypoplastic cusp, freeing of excedentary tissue from the ‘sparrow-nest’ cusp and its reattachment to the plicated annulus). The aneurysm was treated through a reduction pulmonary arterioplasty. Early assessment showed the minimal regurgitation of the valve and a normal diameter PA. The outcome was uneventful, with a stable correction after 44 months of follow-up. To our knowledge, this is the first repair of a quadricuspid pulmonary valve, through tricuspidisation.
Keywords: Congenital heart defect, Pulmonary valve insufficiency, Aneurysm, Pulmonary artery, Cardiac surgical procedure
INTRODUCTION
Pulmonary artery aneurysm (PAA) is a very rare clinical entity. Deterling and Clagett [1] discovered incidences of eight cases in 109 571 consecutive necroptic examinations. The exceptional character of the disease means its traits are still under discussion. There are various aetiologies for PAA [2]; the first descriptions focused on the infectious mechanism (tuberculous or luetic), but more recent studies have found that about half of the PAA cases are associated with congenital heart disease (mostly with patent ductus arteriosus and ventricular septal defects) [3, 4]; the rest are mainly due to vascular wall abnormalities or pulmonary hypertension (PHT).
CASE REPORT
History
Patient T.F., a 16-year old boy had been known to us from the age of 6 months, when a small ventricular septal defect, with an insignificant shunt, was detected. When he was 7-years old, echography proved a spontaneous closure of the defect, but found a moderate pulmonary regurgitation (PR; grade 2/4) with dilation of the main pulmonary artery (PA; 30 mm; Z = +3.4) and annulus (27 mm). The child remained in NYHA class I, but when he was 14-years old, the PA dilation had increased (41 mm; Z = +4) and PR had progressed. Two years later, he returned reporting mild symptoms (NYHA class II); he had a PAA (55 mm; Z = +6), further annular dilation (30 mm), significant PR (grade 3/4) and a dilated right ventricle (RV) (52 mm; Z = +3.8). Cardiac catheterization found normal PA pressures and confirmed the PAA (Fig. 1A).
Figure 1:
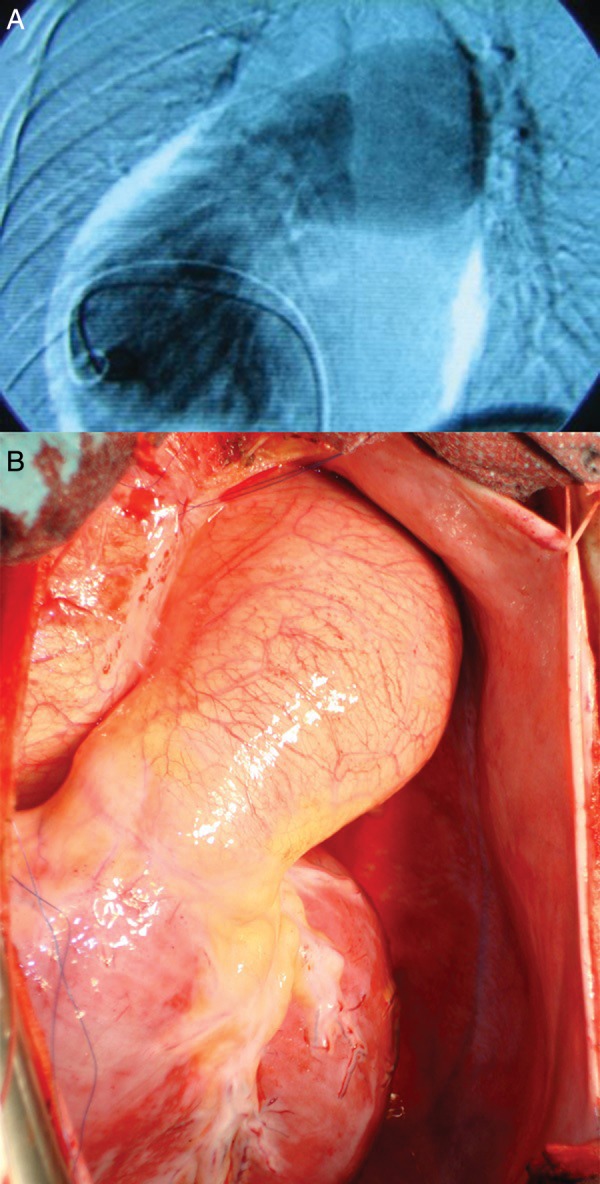
Pulmonary artery aneurysm: (A) angiographic view and (B) intraoperative view.
The rapid increase in the PAA, in conjunction with the significant, symptomatic PR (and consequent RV dilation) were decisive arguments for surgical treatment.
The operation
The operation was performed through a median sternotomy, under normothermic cardiopulmonary bypass, with aortic cross-clamping and cold blood cardioplegia.
Findings
After pericardiotomy, the PAA became obvious (Fig. 1B); it spared the pulmonary bifurcation.
Further evaluation offered a notable surprise: the pulmonary valve had four cusps (‘quadricuspid pulmonary valve’). Two of them were normal, the third was severely dysplastic (some cuspal tissue partly covered its communication with the arterial lumen, giving it a ‘sparrow-nest’ configuration) and the fourth was hypoplastic (some cuspal buds attached to the annulus; Fig. 2A). Thus, the PR was judged as congenital; it was thought to be at the origin of the progressive dilation of the PA.
Figure 2:
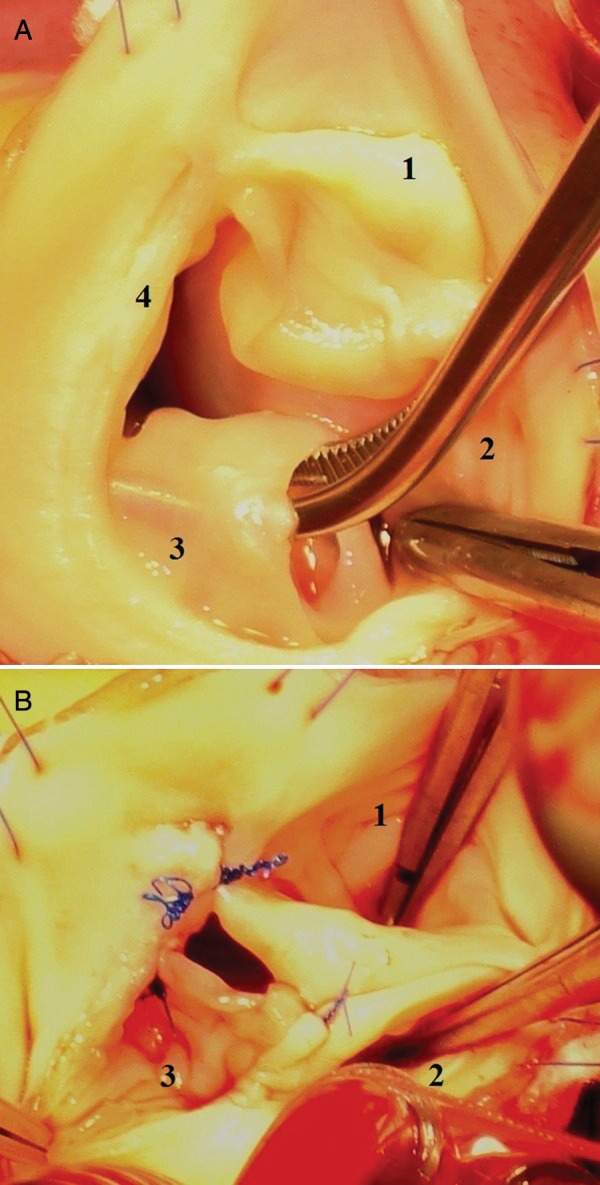
The pulmonary valve (intraoperative view): (A) the initial quadricuspid configuration of the valve (1 and 2: normal cusps; 3: ‘sparrow-nest cusp’; 4: hypoplastic cusp) and (B) the reconstructed pulmonary valve after tricuspidisation (1–3: the cusps after repair).
Technique
The pulmonary valve repair consisted of annulus plication at the level of the hypoplastic cusp insertion (number 4 in Fig. 2A), freeing some excedentary cuspal tissue from the ‘sparrow-nest’ configured cusp (number 3 in Fig. 2A) and its reattachment to the plicated annulus and to the adjacent insertion of the normal cusp (commissural reconstruction). Finally, we obtained a normally configured, tricuspid, continent valve, placed in a normal diameter annulus (Fig. 2B).
Reduction pulmonary arterioplasty was employed for the cure of the PAA. After the longitudinal incision of the aneurysm, two hemioval-shaped pieces of tissue (with a maximal width of 3 cm) were resected from either side of the incision. Finally, after a double-layer continuous suture (polypropylene 4-0), a normalized PA was obtained.
Results
The early postoperative course was uneventful; echography proved a normal diameter PA and minimal PR.
On follow-up (44 months), the correction proved stable, the patient was asymptomatic and the RV diameters had diminished significantly (from 52 to 38 mm).
DISCUSSION
Congenital PR may be one of the possible aetiologies of PAA. It is difficult to identify this preoperatively, as regurgitation may be due to annular dilation (i.e. frequently secondary to a PAA) and also, because echography is less accurate regarding the morphology of the pulmonary valve. Some [5] include such cases in the entity of ‘idiopathic PAA’, which is debatable, as congenital PR may be considered an aetiological factor of the PAA.
Quadricuspid pulmonary valve is a rare congenital abnormality: it was found in 0.2% of 3861 donor hearts [6]. About two-thirds of these valves have a hypoplastic supernumerary cusp [7] which generates PR and eventually may lead to a PAA. As our case shows, the regurgitation is progressive in magnitude, as a consequence of the gradual dilation of the annulus.
Due to the rarity of PAA, there are no clear norms for its surgical treatment. Mastroberto supports surgical indication for all cases that fulfil at least one of the following conditions: (i) reasonable surgical risk (which excludes some of the cases with severe PHT), (ii) progressive increase in aneurysm diameter and (iii) PA dissection [8]. In our case, surgery was indicated as much by the severe PR, generating symptoms and RV dilation, as by the progressive increase of the PA diameter.
Correction of the PR might be done either through pulmonary valve replacement, or better, whenever possible, through valve repair. As the anatomy seemed favourable (dilated annulus, two normal cusps and the third with an excess of cuspal tissue), we opted for an unusual reconstruction of the pulmonary valve (tricuspidisation of the quadricuspid valve), which proved to be effective. Tricuspidization of quadricuspid aortic valves has been reported by Schmidt et al. [9], others have reported the bicuspidization of regurgitant tricuspid pulmonary valves [5], but, to our knowledge, this is the first report of a tricuspidization of a pulmonary quadricuspid regurgitant valve.
Various techniques may be applied for the cure of PAA: resection of the aneurysm followed by PA reconstruction with a tube (PTFE, Dacron, a homograft or pericardium) [10], aneurysmoraphy, partial excision followed by patch reconstruction or reduction through a pulmonary arterioplasty [4].
We treated the aneurysm through an arterioplasty, as the PAA was secondary to a congenital PR, and concomitant structural abnormalities of the arterial wall seemed less probable. Our attitude is sustained by the Mayo Clinic experience, which showed that reduction through arterioplasties is as efficient as resection-interposition techniques in the cure of PAA [4]. It seems to be a convenient technique, whenever the pulmonary pressure is normal and there is no suspicion of structural abnormalities of the arterial wall. Though the long-term durability of the correction is yet to be proven, the medium-term result was very good, the echographic assessment showing a stable repair after 44 months of follow-up.
Conflict of interest: none declared.
REFERENCES
- 1.Deterling RA, Jr, Clagett OT. Aneurysm of the pulmonary artery: review of literature and report of a case. Am Heart J. 1947;34:471–99. doi: 10.1016/0002-8703(47)90527-9. [DOI] [PubMed] [Google Scholar]
- 2.Bartter T, Irwin RS, Nash G. Aneurysms of the pulmonary arteries. Chest. 1988;94:1065–75. doi: 10.1378/chest.94.5.1065. [DOI] [PubMed] [Google Scholar]
- 3.Nair KKS, Cobanoglu AM. Idiopathic main pulmonary artery aneurysm. Ann Thorac Surg. 2001;71:1688–90. doi: 10.1016/s0003-4975(00)02568-6. [DOI] [PubMed] [Google Scholar]
- 4.Deb SJ, Zehr KJ. Shields RC idiopathic pulmonary artery aneurysm. Ann Thorac Surg. 2005;80:1500–2. doi: 10.1016/j.athoracsur.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Muthialu N, Raju V, Muthubaskaran V, Chandrasekar P, Muralidharan S, Kuppanna PJ. Idiopathic pulmonary artery aneurysm with pulmonary regurgitation. Ann Thorac Surg. 2010;90:2049–51. doi: 10.1016/j.athoracsur.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 6.Jashari R, Van Hoeck B, Goffin Y, Vanderkelen A. The incidence of congenital bicuspid or bileaflet and quadricuspid or quadrileaflet arterial valves in 3,861 donor hearts in the European Homograft Bank. J Heart Valve Dis. 2009;18:337–44. [PubMed] [Google Scholar]
- 7.Davia JE, Fenoglio JJ, DeCastro CM, McAllister HA, Jr, Cheitlin MD. Quadricuspid semilunar valves. Chest. 1977;72:186–9. doi: 10.1378/chest.72.2.186. [DOI] [PubMed] [Google Scholar]
- 8.Mastroroberto P, Chello M, Zofrea S, Del Negro G, De Francesca F, Maltese G. Correspondence: pulmonary artery aneurysm. Ann Thorac Surg. 1997;64:585–6. [PubMed] [Google Scholar]
- 9.Schmidt KI, Jeserich M, Aicher D, Schäfers HJ. Tricuspidization of the quadricuspid aortic valve. Ann Thorac Surg. 2008;85:1087–9. doi: 10.1016/j.athoracsur.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Vistarini N, Aubert S, Gandjbakhch I, Pavie A. Surgical treatment of a pulmonary artery aneurysm. Eur J Cardiothorac Surg. 2007;31:1139–41. doi: 10.1016/j.ejcts.2007.03.014. [DOI] [PubMed] [Google Scholar]


