Table 2.
Representative benzylic-alkenyl cross-couplingsa
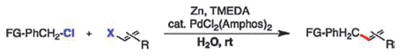 | |||
|---|---|---|---|
| Entry | Product (Yield)b | Entry | Product (Yield)b |
| 1 |
2 (82%) |
2 |
3 (91%) |
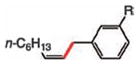 |
|||
| 3 | 4 R = CF3 (84%) | 5 | 6 R = H (95%) |
| 4 | 5 R = Cl (89%) | 6 | 7 R = OMe (81%) |
| 7 |
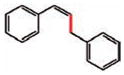 8 (90%) |
8 |
9 (85%) E/Z 83/17c |
| 9 |
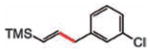 10 (77%) E/Z 92/8d |
10 |
 11 (95%) |
| 11 |
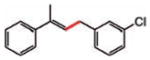 12 (92%) |
12 |
13 (96%) |
| 13 |
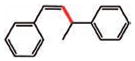 14 (83%) |
14 |
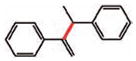 15 (79%)e |
| 15 |
16 (82%) |
||
Conditions: benzylic chloride (2–3 mmol), alkenyl halide (X = I, for entries 1–6, 10, 12, 15, and X = Br for entries 7–9, 11, 13–14; 1 mmol), zinc dust (3–4 mmol), PdCl2(Amphos)2 (0.02 mmol), degassed water (3 mL), rt, 6 h.
Isolated yield.
From commercially available β-bromo-styrene, E/Z = 87/13.
From commercially available (2-bromovinyl)trimethylsilane, E/Z = 93/7.
From commercially available 90% technical grade a-bromostyrene.
