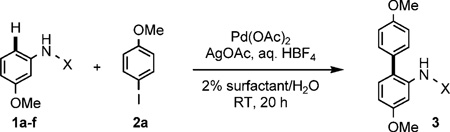
Table 1.
Optimization of C–H arylations at room temperature.[a]
 | ||||
|---|---|---|---|---|
| Run | X | Surfactant | Yield [%] | |
| 1 | COMe | 1a | PTS | trace |
| 2 | COiPr | 1b | PTS | trace |
| 3 | COtBu | 1c | PTS | 24 |
| 4 | CSNMe2 | 1d | PTS | 0 |
| 5 | COCF3 | 1e | PTS | trace |
| 6 | CONMe2 | 1f | PTS | 67(0,[b] 0,[c] 0,[d] 60[e]) |
| 7 | CONMe2 | 1f | Triton X-100 | 73 (47,[f] 20[g]) |
| 8 | CONMe2 | 1f | Solutol | 65 |
| 9 | CONMe2 | 1f | Brij 35 | 76 |
| 10 | CONMe2 | 1f | Brij 30 | 72 |
| 11 | CONMe2 | 1f | TPGS | 73 |
| 12 | CONMe2 | 1f | Cremophor EL | 40 |
| 13 | CONMe2 | 1f | none | 35 |
Conducted at room temperature for 20 h in 2 wt% surfactant/water with 10 mol% Pd(OAc)2, AgOAc (2 equiv), aq. HBF4 (5 equiv), 1 (0.25 mmol), and 2 (2 equiv).
AcOH (instead of HBF4).
HCl.
TFA.
TsOH.
1.2 equiv AgOAc.
3 mol% catalyst.
