TABLE 1. Optimizing Reaction Conditions.
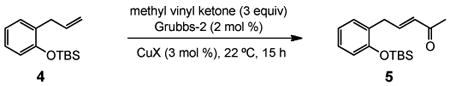 | |||
|---|---|---|---|
| entry | CuX | solventa | conversion (%)b |
| 1 | CuCN | CH2CI2 | 24 |
| 2 | CuOAc | CH2CI2 | <5 |
| 3 | Cu(OTf)2 | CH2CI2 | 0 |
| 4 | Cu(BF4)2 | CH2CI2 | 15 |
| 5 | Cu(NO3)2 | CH2CI2 | 0 |
| 6 | Cu(CIO4)2 | CH2CI2 | 22 |
| 7 | Cu(CF3COO)2 | CH2CI2 | 0 |
| 8 | Cu(CH3CN)4PF6 | CH2CI2 | 27 |
| 9 | CuCI | CH2CI2 | 35 |
| 10 | CuBr | CH2CI2 | 43 |
| 11 | CuI | CH2CI2 | 64 |
| 12 | - | CH2CI2 | 45 |
| 13c | CuI | CH2CI2 | 63 |
| 14d | CuI | CH2CI2 | 68 |
| 15 | CuI | Toluene | 64 |
| 16 | CuI | THF | 70 |
| 17 | CuI | Et2O | 71 |
| 18e | CuI | Et2O | 76 |
| 19f | CuI | Et2O | 85 |
| 20f | - | Et2O | 43 |
| 21g | CuI | Et2O | >99 (98)h |
Reaction run in 0.5 m solution.
Based on 1H NMR.
Using 6 mol % CuI.
Using 3 mol % Grubbs-2 and 6 mol % CuI.
Reaction run in 0.2 m solution.
Reaction run in 0.1 m solution.
At 35 °C for 3 h; without CuI; yield = 57%
Isolated yield.
