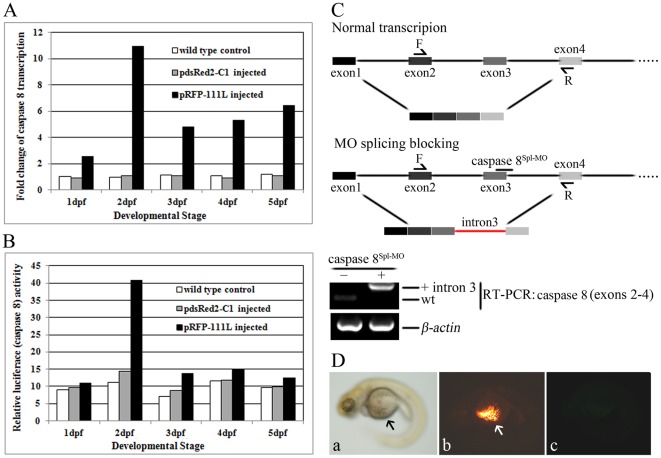
Figure 4. ISKNV ORF111L-induced apoptosis was mediated by caspase 8.
(A) The caspase 8 expression was tested by RT-qPCR assay. The expression level of β-actin was set as 1, and values were normalized to the corresponding β-actin values to determine the relative copy number. All data are presented as means from three individual injection experiments. (B) The caspase 8 activity was tested in wild type, pdsRed2-C1- and pRFP-111L-injected embryos. (C) Knockdown efficiency of caspase 8Spl-MO in zebrafish embryos. Schematic representation of normal transcription of caspase 8 and morpholino splicing blocking of caspase 8 was shown. The caspase 8Spl-MO interferes with splicing junction at exon 3/intron 3, resulting in retention of the intron 3. The retention of intron 3 resulted in a frame-shift and the truncation of protein translation. Knockdown effect of caspase 8Spl-MO was tested in by RT-PCR. Forward primer is located in exon 2, and the reverse primer is located in exon 4. Result showed that caspase 8Spl-MO strongly depletes the wild type caspase 8 mRNA and had a high gene knockdown effect. (D) Caspase 8 knockdown effectively blocked ISKNV ORF111L-induced apoptosis. The caspase 8Spl-MO and pRFP-111L were co-injected into 1–2 cell stage embryos. The yolk sac of embryo was healthily developed (panel a). The expression of RFP-111L fusion proteins were clear (panel b), and no obvious apoptosis signal were found in TUNEL assay (panel c), indicating that knockdown the expression of caspase 8 could effectively block ISKNV ORF111L-induced apoptosis. Embryos shown above are the typical phenotype in three independent experiments.