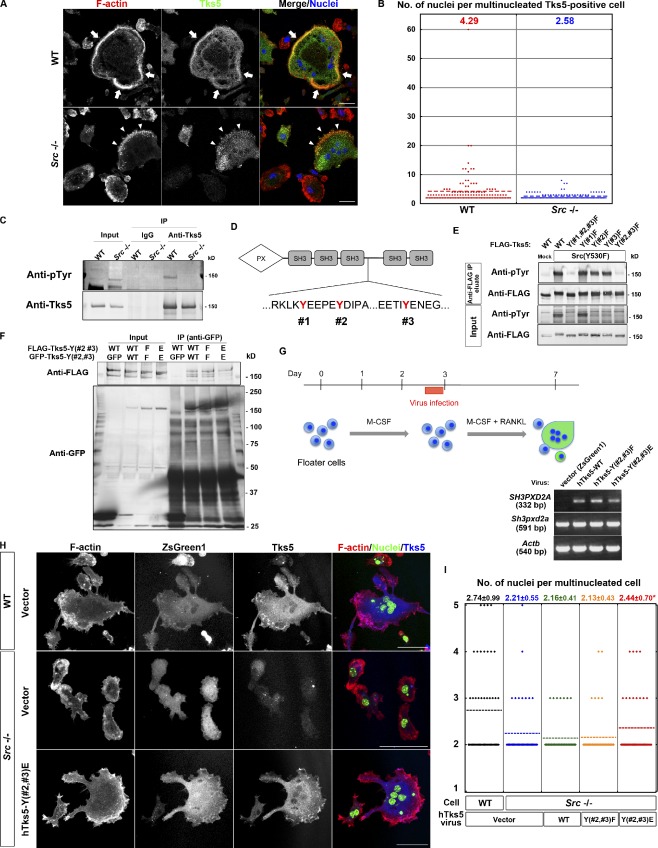
Figure 4.
Phosphorylation by Src is required for Tks5 to induce circumferential podosomes and osteoclast fusion. (A) Representative images of multinucleated osteoclasts differentiated from spleen macrophages of WT or Src−/− mice. The cells were stained with rhodamine-phalloidin to visualize F-actin, with antibodies to Tks5, and with DAPI to visualize nuclei. Mononuclear cells tended to express little Tks5, whereas Tks5 was abundant in multinuclear cells. Arrows indicate a sealing belt, and arrowheads indicate actin puncta formed at the cell periphery. Bars, 25 µm. (B) Quantification of the number of nuclei per cell in multinucleated Tks5-positive osteoclasts differentiated from spleen macrophages of WT or Src−/− mice. More than 100 cells were counted, and the mean values are indicated. (C) Lysates of osteoclasts differentiated from spleen macrophages of WT or Src−/− mice were subjected to immunoprecipitation with antibodies to Tks5 or control IgG. The resulting precipitates, as well as the original cell lysates (Input), were then subjected to immunoblot analysis with antibodies to phosphotyrosine (pTyr) or to Tks5. (D) The amino acid sequences of hTks5 containing putative Src phosphorylation sites (#1, #2, and #3). (E) NIH 3T3 cells were cotransfected with a vector for FLAG-tagged hTks5-WT or mutants thereof with the indicated tyrosine residues replaced with phenylalanine together with a vector for an active form of Src, Src(Y530F), or the corresponding empty vector (Mock). Cell lysates were subjected to immunoprecipitation with agarose-conjugated antibodies to FLAG, and the precipitated proteins were eluted with the FLAG peptide. The eluted proteins, as well as the original cell lysates (Input), were subjected to immunoblot analysis with the indicated antibodies. (F) NIH 3T3 cells coexpressing GFP-tagged and FLAG-tagged forms of hTks5-WT or the indicated phenylalanine or glutamate substitution mutants were subjected to immunoprecipitation with antibodies to GFP. The resulting precipitates, as well as the original cell lysates (Input), were then subjected to immunoblot analysis with the indicated antibodies. See also Fig. S2 A. (G, top) Protocol for virus infection during osteoclastogenesis from primary spleen macrophages. (bottom) The infected osteoclasts were subjected to semiquantitative RT-PCR analysis to confirm the expression of hTks5 constructs. (H) Representative images of virus-infected (ZsGreen1 positive) osteoclasts differentiated from either WT or Src−/− spleen macrophages. The cells were stained with rhodamine-phalloidin (red), antibodies to Tks5, and DAPI (green). Bars, 50 µm. (I) Quantification of the number of nuclei per cell in multinucleated osteoclasts with ZsGreen1 expression. Means ± SD from three independent experiments are indicated. *, P < 0.05 versus Src−/− cells with the vector (Student’s t test). See also Fig. S2 B and Videos 8 and 9. IP, immunoprecipitation.